Incoherent-Light Hazard Classification for Medical Devices
Incoherent-Light Hazard classification standards that may pertain to your medical device include: ISO 60601-2-57, IEC 62471, ANSI RP-27-20, ISO 15004-2, ANSI Z80.36, ISO 10936-2, ISO 10939, and so on…
In this blog post, I’ll examine certain commonalities and points of potential confusion of the “big 4” incoherent-light-source safety standards: IEC 62471 and ANSI RP-27-20 for “general biophotonic hazards”, and ISO 15004-2 and ANSI Z80.36 for hazards specific to ophthalmic devices and the patients to which they are applied.
The intended market and indications for use of your medical device will determine which standard or combination of standards apply to your product. These standards share certain common elements – but also have important differences.
It’s worth noting that these standards are the work of committees, sometimes with overlapping membership. Each committee is informed by previous versions of the standard, work on other standards, recent research, and safety updates provided by the International Committee on Non-Ionizing Radiation Protection[1][2][3].
However, each distinct committee needs to achieve its own internal consensus on hazard limits that finds the right balance between practicality, incorporation of new research findings, continuity with previous versions of the standard, and each member’s personal and professional opinion about said balance.
For these reasons, the standards often differ in their details. For example, as discussed in its Foreword, the very existence of ANSI Z80.36 arose from an inability to reach a consensus within the ISO 15004-2 working group.
Generally, you should expect broad agreement of risk categories and manner of assessment, but you should not expect complete agreement in detail. I’ll highlight some other differences below.
If you’re faced with assessing biophotonic risk for your medical device, my advice is to start by consulting the ICNIRP guidelines – though they can sometimes seem disjointed, they provide good background on the potential physiological damage mechanisms and other considerations at play.
Next, consult your Regulatory Affairs specialist to ensure you understand which standards may or may not apply to your medical device.
Finally, sit down with one applicable standard, and read through bit-by-bit in small chunks. As well as studying the limits, read the sections on measurement methodology – to make the calculations more concrete. Repeat for other applicable standards.
Though these standards can seem daunting, contradictory, and sometimes disorganized, they do share broad similarities. Careful application will lead to a better assessment of the risks produced by your medical device.
Incoherent-Light Hazard Classification Applicability and Risk Categorization
It is worth noting at the outset that IEC 62471 and ANSI RP-27-20 are lamp standards, meant to apply to everything from desk lamps to display lighting, from overhead lights to arc lamps. In the context of medical devices, they allow assessment of general biophotonic risk to clinical staff and – in the case of non-ophthalmic devices – to the patient.
In contrast, the ophthalmic standards ISO 15004-2 and ANSI Z80.36 assess risk to the patient. As well – and unlike the previous two standards – these ophthalmic standards classify risk level due to both incoherent and coherent radiation incident on the eye.
In any event, each of the standards expresses the risk level associated with the medical device based on the time required to reach a hazardous level of exposure. The lamp standards classify light sources as either exempt (very low risk), Group 1 (low/very-low risk), Group 2 (moderate/low risk) or Group 3 (high risk). The ophthalmic standards break devices into either Group 1 (no potential light hazard) or Group 2 (potential light hazard).
Hazard Types
All the standards consider light-induced biological changes that are either photochemical in nature or thermal. Photochemical damage/hazard is proportional to the total absorbed radiant exposure (i.e. dose per unit area) – at least for exposures of up to several minutes or more. At longer times, the hazard limits become dose-rate limited.
In contrast, the thermal hazard depends on the temperature that tissue reaches under exposure. This, in turn, depends on the detailed balance between effective beam spot size (absorbed energy) and the thermal conduction of heat away from the affected region (rate of energy dissipation). So, for example, small spots cool more-quickly than larger ones.
At the same time, in the case of pulsed exposures, if the pulse ends before any of the heat has a chance to diffuse away, then all the deposited light energy leads to local heating. For these reasons, the formulas for thermal hazards are generally more complex than those for photochemical ones.
For thermal damage of the retina, for example, the spot-size dependence is explicitly captured in the exposure limits – either explicitly via retinal image size (ophthalmic standards ISO 15004-2 and ANSI Z80.36) or implicitly via source angular size (lamp standards IEC 62471 and ANSI RP-27-20).
Limitations on said quantities capture image spread due to natural eye motion on the small-retinal-image end, and long-term eye motion or saturation of spot-size damage dependence on the large-retinal-image end. For pulsed sources, the temporal dependence of heating is explicitly captured in the hazard limit – usually with a functional form that is, itself, time-dependent.
Wavelength-dependence of damage risk is captured in the standards by spectral weighting functions. These functions encapsulate physiological spectral variations in damage probability due to varying absorption and/or damage mechanism. For historical reasons, these weighting functions may have a peak value larger than 1 – thus enhancing the impact of certain wavelengths (e.g. the thermal risk to the retina in ANSI Z80.36 and IEC 62471).
A complementary manner of categorizing the hazards is retinal vs. non-retinal hazards. The damage mechanisms and dynamic considerations remain the same, but some of the standards express the retinal hazard limits in terms of radiance Ls at the lens (W/[cm²∙sr]) rather than the irradiance Er (W/cm²) at the retina.
This is – again – for the convenience of the assessor: most test technicians are reluctant to insert a power meter into their eyeball! The relationship between radiance at lens and irradiance at retina is then obtained by using a standard theoretical eye model[4] and an assumed pupil diameter:
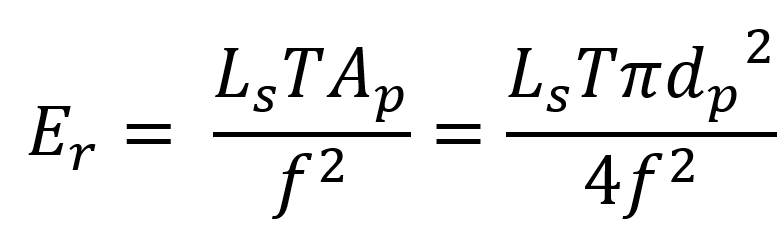
In the above T is the transmissivity of the eye, dp the pupil diameter (usually conservatively taken to be 7 mm), Ap the pupil area, and f is the effective focal length of the eye (normally taken to be 17 mm, per the Gullstrand model eye). The limits can then be translated from one set of units to another as convenient (or confusing!).
Each of the standards differs slightly in its naming convention: Table 1 groups the “equivalent” hazards for the 4 standards. Also shown are the applicable hazard types (photochemical/thermal) and the physiology to which they apply (retinal/anterior segment/other).
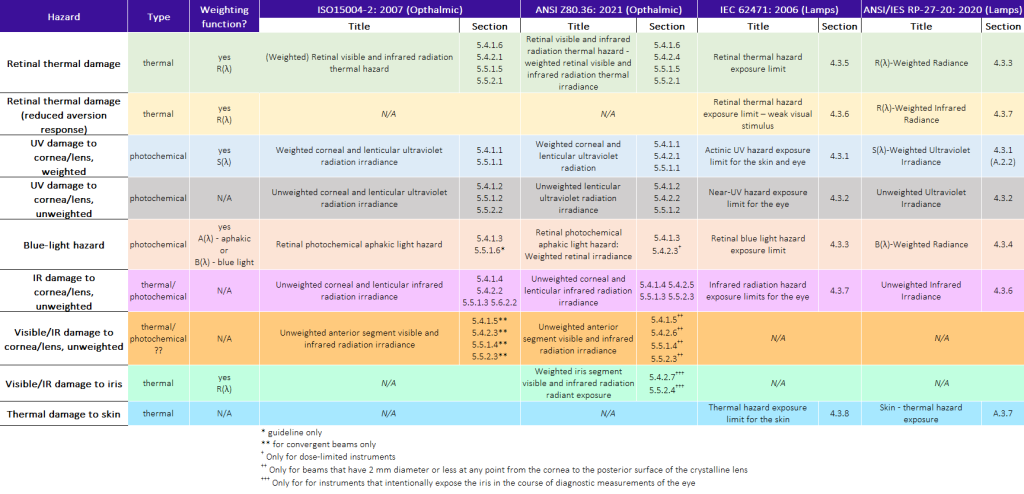
Differences in Incoherent-Light Hazard Classification Standards
I put the word equivalent in quotes in the above paragraph because the detailed hazard assessment may differ from standard to standard. To take some concrete examples, consider the ophthalmic standards ISO 15004-2 and ANSI Z80.36:
- The wavelength interval over which weighted UV cornea/lens hazards ES-CL are assessed is identical between the standards (250 nm to 400 nm), and the weighting-function values S(λ) are effectively identical. However, the Group 1 limit values for CW sources have different values between the two standards, and the Group 2 CW limits are dose-based for ISO 15004-2, but rate-based for ANSI Z80.36.
- Similarly, the Group 1 CW unweighted anterior-segment cornea/lens hazard limit EIR-CL is not only lower in ISO 15004-2 than in ANSI Z80.36, but the hazard is evaluated over a much larger wavelength range (770 nm to 2500 nm for the former, versus 915 nm to 2500 nm for the latter).

- The ANSI standard has an additional, pulse-duration/period-dependent limit-reduction factor Cp in the expression for Group 2 pulsed retinal thermal hazard that the ISO standard does not have.
- ANSI Z80.36 (5.5.2.1): HlimVIR-R Group 2 pulsed = (10∙t3/4∙Cp/dr) J/cm² (for 3 μs < t < 5000 s)
- ISO 15004-2(5.5.2.1): HlimVIR-R Group 2 pulsed = (10∙t3/4/dr) J/cm²
Unfortunately, if you’re considering a device’s safety by multiple standards, the only recourse is a detailed, methodical calculation of classification by each of the applicable standards.
Even comparing CW and pulsed limits within a given standard can seem inconsistent at first glance. For example, the Group 1 CW limit (5.4.1.4) in ANSI Z80.36 for EIR-CL is given as 60 mW/cm², and the preamble (Sec. 5.2) states that “the Group 1 limit values given in 5.4 are based upon a 5000-second (1.4-hour) exposure.” However, if we take 5000 s as the exposure time and calculate the accrued radiant exposure H5000 s, Group 1 CW, we obtain
H5000 s, Group 1 CW = (60 mW/cm²)∙(5000 s) = 300,000 mJ/cm² = 300 J/cm²
On the other hand, if we treat the whole 5000 s exposure as a long single pulse, the corresponding Group 1 pulsed limit would be (5.4.2.5)
Hlim, IR-CL Group 1 pulsed (5000 s)= 1.8∙(5000 s)1/4 J/cm² = 15 J/cm².
There appears to be an obvious disjoint! However, it is likely the case that an implicit (or obscure) additional assumption was made by the committee in coming up with the pulsed-dose (or integrated CW- rate) limit. Again, the only recourse is detailed, methodical calculation by the most-applicable section of the standard.
Conclusion
In conclusion, the Incoherent-Light Hazard Classification eye-safety standards applicable medical devices all work to prevent injury to patients and clinic staff. However, the individual limits and detailed calculations reflect the best judgement of the particular st of experts authoring each study. You can apply the general principles of one standard to interpreting another, but in the end you must run through the detailed calculations for each and every document that applies to your medical device.
I hope this blog will serve as a useful introduction to that effort!
Images:
Title Image: https://pixabay.com/photos/eye-test-ophthalmology-man-eye-care-5028103/
Image 2: https://www.flickr.com/photos/shannonpatrick17/3907957458
Image 3: https://commons.wikimedia.org/wiki/File:Laser_King_2.jpg
Table 1: StarFish Medical
Brian King is Principal Optical Systems Engineer at StarFish Medical. Previously, he was Manager of Optical Engineering and Systems Engineering at Cymer Semiconductor, Brian was an Assistant Professor at McMaster University. Brian holds a B.Sc in Mathematical Physics from SFU, and an M.S. and Ph.D. in Physics from the University of Colorado at Boulder.
[1] ICNIRP Guidelines on Limits of Exposure to Ultraviolet Radiation of Wavelengths Between 180 nm and 400 nm (Incoherent Optical Radiation). Published in: Health Physics 87 (2), 171 (2004).
[2] ICNIRP Guidelines on Limits of Exposure to Incoherent Visible and Infrared Radiation. Publish in: Health Physics 105 (1), 74 (2013).
[3] Adjustment of guidelines for exposure of the eye to optical radiation for ocular instruments: statement from a task group of the International Commission on Non-Ionizing Radiation Protection (ICNIRP), Published in: Applied Optics 44 (11), 2162 (2005)
[4] All the major standards use the Gullstrand model eye: Gullstrand A, Die Dioptrik des Auges. Helmholtz, Handbuch der physiologischen Optik, 3rd ed. 1; 1909.
In many cases, optical scattering plays a strong role in the propagation of optical radiation. One common approach to simulating scattering is the Monte Carlo technique.
