Bio Break: Finding the Path to MedTech Innovation with the Pathfinder Program
Pathfinder Program MedTech Innovation
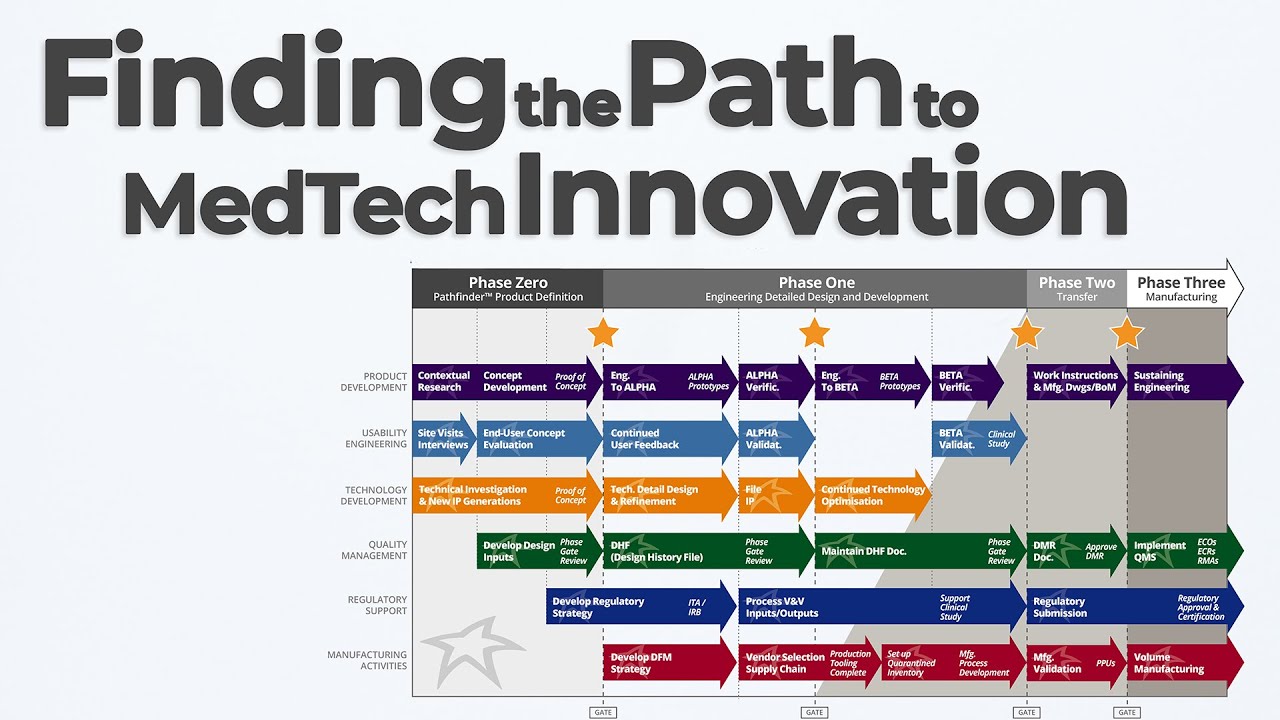
In this Pathfinder Program MedTech Innovation episode of Bio Break, Nick Allan and Joris explore one of the most dynamic early-phase services at StarFish Medical: the Pathfinder Program. If you’re a medtech innovator with a promising concept or prototype, Pathfinder helps you identify the right path forward—before you invest millions in development.
Nick explains that Pathfinder projects focus on structured, interdisciplinary exploration to de-risk early innovation decisions. Whether it’s a new diagnostic platform or a novel therapeutic device, these short, intensive engagements are designed to define your target product profile, investigate regulatory and IP strategy, and uncover unmet user needs—all before a single part is manufactured.
The episode discusses how the Pathfinder team collaborates across human factors, industrial design, systems engineering, and sometimes even early prototyping to assess feasibility and market alignment. The outcome? A comprehensive Pathfinder Report. This document helps clients understand competitive positioning, reimbursement potential, freedom to operate, and how to prioritize the product’s unique value in the clinical space.
Joris and Nick also reflect on how Pathfinder enables clients to avoid costly pivots later in the process. By bringing in industrial designers and regulatory experts early, teams can explore device form factor, usability constraints, and even conduct basic visualizations or 3D-printed proofs-of-concept.
Whether you’re preparing for a funding round, seeking to understand your market fit, or building a go-to-market strategy, the Pathfinder Program can give your team clarity and confidence. Tune in to see how this process sets the foundation for successful medical device commercialization—and why it’s one of the most rewarding project types at StarFish.
Learn more about StarFish Medical.
Related Resources

For manufacturers of novel devices that can make a significant impact to patient health, the goal of the program is to offer a path to streamlined and potentially faster market entry without sacrificing the rigour around ensuring safety and performance.

When I was starting out in medical devices, the discussion focused on the possibility of an internet of things and the promise of “big data” about everything.

With the release of ISO 14644-5:2025, Cleanrooms and associated controlled environments, Part 5: Operations, the standard places increased emphasis on operational discipline, human factors, and contamination control behaviour.

This article outlines the core elements of the PCCP framework, the types of modifications it applies to, and how the FDA expects manufacturers to use it in practice.