Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Ariana Wilson and Mark Drlik break down medical vs wellness devices and explain why two products with identical hardware can fall into completely different regulatory categories.
-

Ariana Wilson sits down with Mark Drlik to unpack why reprocessing is often one of the hardest challenges engineers face during development.
-

Nick and Nigel walk through how teams decide between ethylene oxide, E-beam, and gamma radiation sterilization.
-

In MedTech, success rarely comes from invention alone. Plenty of promising technologies make it through verification and early clinical work, only to stall when the team tries to turn them into something buildable.
-

Nick walks through a practical Teflon tape lesson that came from real work supporting a mechanical test rig.
-
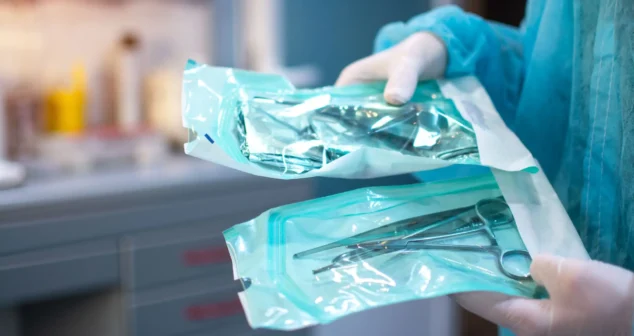
Most sterile medical devices begin their journey long before anyone thinks about sterilization. Teams focus on function, usability, materials, and suppliers, then discover that sterilization constraints can reshape many of those early decisions.
-

After years of working with founders and technical teams, I have learned that early design missteps rarely come from engineering flaws. More often than not, they come from missing conversations.
-

Medtech founders operate with more constraints than most sectors. You are responsible for deep technical problem solving, high-stakes decisions, regulatory navigation, investor conversations, and a constant stream of operational tasks.
-

Every MedTech startup begins with a hypothesis, an idea that could transform patient outcomes, simplify delivery of care, or improve how clinicians diagnose and treat patients.