The user experience (UX) design process for medical devices: research
The UX design process for medical devices places emphasis on safety and documentation. Designers are provided standards such as IEC 62366-1 and IEC 62366-2 to guide us in the application of usability engineering to medical devices. I will be referencing them a lot.
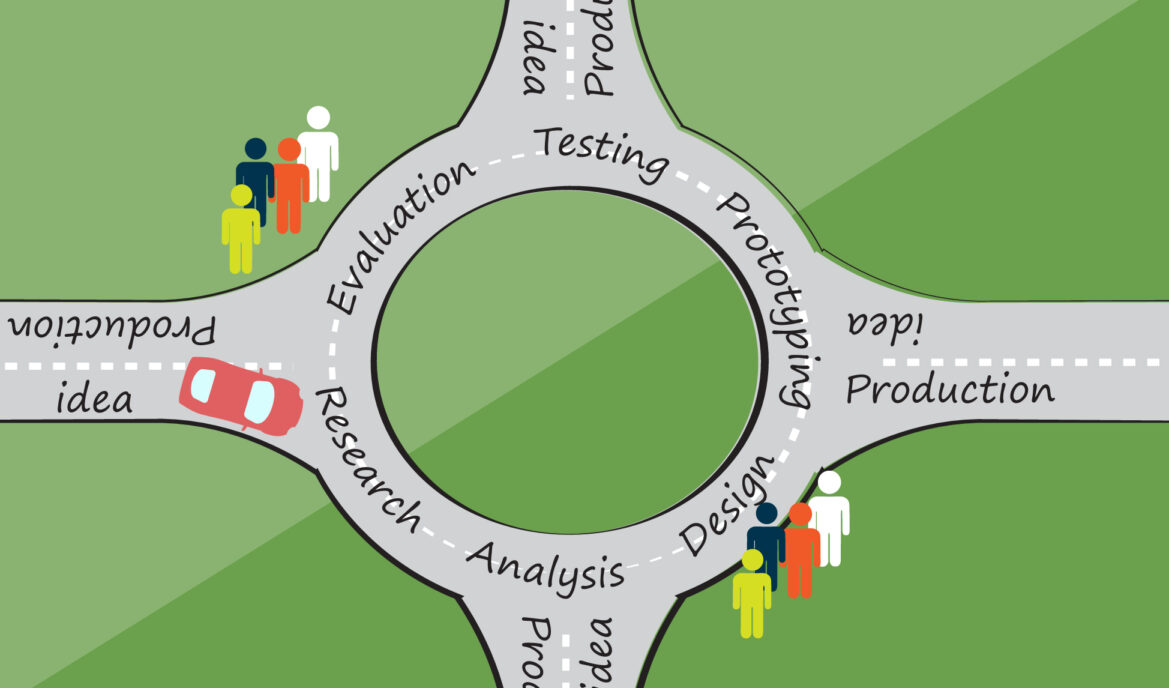
Research is the first subject of my three part blog series on the User Experience (UX) design process for medical devices. While reading this blog, keep in mind that I am laying out steps but UX design is more like a traffic circle. You keep driving around until you finally know for sure which direction to go.
Preliminary Use Specifications
You start off with an idea. “I want to design a blood pressure monitor that’s accessible from my phone.” With that start, you can explore “…important characteristics related to the context of use of the medical device” as defined by IEC 62366-1. Preliminary use specifications are gathered from readily available data to plan and conduct appropriate user research. They start as a draft and are constantly updated as you gain new knowledge from user research. Eventually, they will specify your patient population, user profiles, which part of the body or tissue the device will be interacting with, the use environment, and operating principle.
User Research
From your preliminary analysis, you might have discovered who your general user groups are, their anticipated user tasks, and the typical use environment. Now you can plan and conduct user studies befitting your needs: Is your goal to figure out the proper use of current blood pressure monitors? Or to amass the creative thoughts of your users?
If it’s the former, behavioural research is the way to go. This includes usability tests, tasks analysis, and card sorting. You’re able to observe how people interact with a product, capturing passive information and honest data. However, you don’t get to hear what your users really think of your product.
If it’s the latter, attitudinal research is your choice. This includes surveys, interviews, and focus groups. You’re able to listen to your users’ thoughts and opinions. The disadvantage is that it requires honesty and in some cases, what people say does not correlate with what people want and actually do.
How about achieving the best of both worlds? Contextual interviews let us follow medical specialists in their work environment while you ask questions. If you’d like to know more about research methods in detail check out What & Why of Usability.
In the research phase, your mind needs to be a blank canvas and open to any and every sort of information that you’ll be gathering during your studies. Discover the personality of each user group and take notice of the problems they tend to passively ignore. No matter how small the detail, treat them all as a potential venture and you’ll often find them to be the selling points. Empathy is the key to seeing from the user’s perspective and to feeling what they feel. Be their best friend.
User Profiles (Personas) and Use Scenarios
Once user research goals have been met, it’s time to extrapolate the data to form user profiles and use scenarios. IEC 62366-1 defines user profiles as “a summary of the mental, physical and demographic traits of an intended user group, as well as any special characteristics such as occupational skills, job requirements and working conditions, which can have a bearing on design decisions”. There are generally a handful of user profiles, each representing a user group and they should be defined in levels of hierarchy. For a blood pressure monitor, you’ll need a user profile for the patient, the nurse, the physician and the technician. This acts as a reference to fall back when making design decisions. Does it fit your user profiles? No? Scrap it.
IEC 62366-1 defines use scenarios as “specific sequence of tasks performed by a specific user in a specific use environment and any resulting response of the medical device”. These scenarios specify a use case and are written in the perspective of a user group. These are done to clearly point out product requirements as they lay out the user’s goals, their tasks to complete those goals, and any required action the product needs to perform in order to achieve those goals. These are typically done with an analogous device.
Product Requirements
So you don’t spend precious time re-inventing the wheel, researching products that currently exist on the market is an important step. This can help you brainstorm on desirable and undesirable features. This also helps in conducting a competitive analysis to see what’s possible that will set your device apart from other products on the market. It doesn’t necessarily have to be of the exact product you plan on creating, it could be an analogous device.
For example, if you’re trying to create a blood pressure monitor for home health care, your research on fitness trackers and their associated mobile applications might lead you to explore cloud based systems to share information. You’re not designing the product yet, you’re merely brainstorming, benchmarking, and conducting a trend analysis so you can start writing down the product requirements and use specifications.
Combine these requirements with ones gathered from your use scenarios and compare them with the requirements from your user profiles. You’ll have a strong start on a product requirements document. A product requirements document is very fluid, and can always change throughout the entire design process, much like everything else in the UX design process.
Risk analysis
Throughout the process, you’re analysing potential risks and hazards that your product might have. This is part of the risk management process described in ISO 14971. Once your product requirements begin, so should your risks analysis. More detail on the risk analysis will be covered in blogs to follow.
The next phase of the UX design process for medical devices involves conceptualizing. I will detail the process in which to provide a proper information architecture and workflow, and how to illustrate them using wireframes.
Kimberly Nguyen is a former Industrial Designer at StarFish Medical. She works on a variety of Medical Device projects and a strong proponent of carefully researched UX design.
Images: StarFish Medical
