The Conformité Européenne (CE) mark is a common sight on products in North America, and Europe. However the China Export mark and CE mark are easily confused, which is understandable because they look almost identical. This similarity is something which has been acknowledged by the EU parliament. In this blog differences between the CE vs China Export Mark are explained along with tips to avoid costly mistakes.
If you’ve taken a look at the safety and specification notes on many products you’ve no doubt seen a mark that looks like the one pictured above on many of them. They frequently appear alongside the common UL (Underwriters Laboratory), FCC (Federal Communications Commission), or CSA (Canadian Standards Association) marks that appear on many products in North America. Given the vast difference in the meaning of these symbols, engineers need to be able to tell them apart. This blog explains the meaning of both marks and provides a simple way to differentiate them.
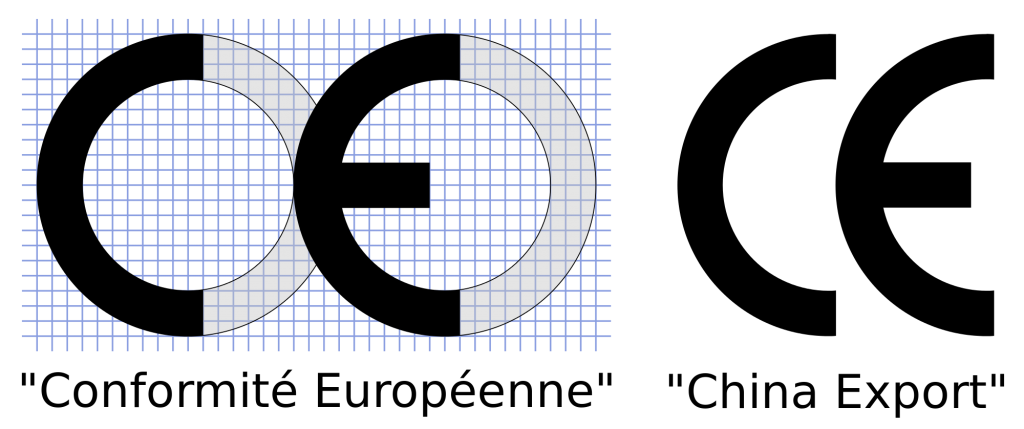
The Conformité Européenne (CE) Mark
The CE Mark is required on certain products that are sold in the European Economic Area (EEA). There are a number of compliance categories which cover different products. For most products companies don’t need to seek external inspections to use the mark. For products that require the mark, companies only need to ensure their product meets the applicable standards through testing, provide a declaration, and then place the mark on their product. Certain categories such as medical devices, require testing by an accredited third party called a Notified Body.
In order for a medical device to receive a CE mark, the process is more intensive. Designers must submit samples and technical documents for external inspection by any of a number of Notified Bodies. These bodies review the product documentation, and depending on the requirements will review test reports or conduct their own testing to ensure a product complies with the regulations.
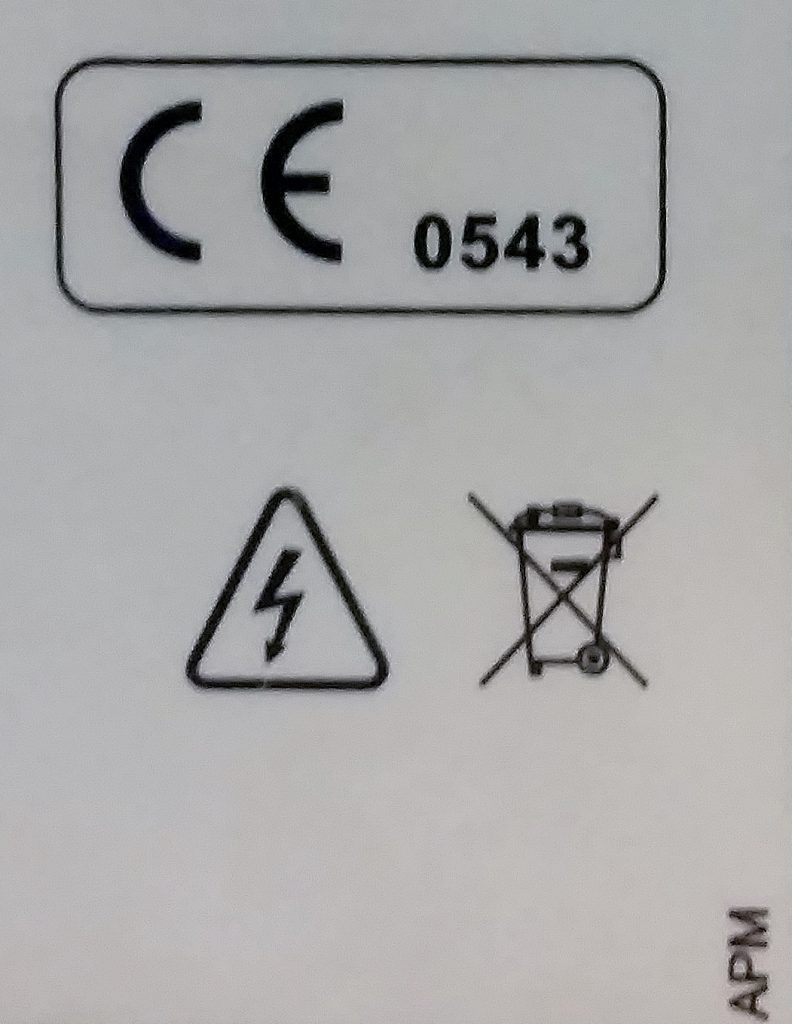
The CE mark on medical devices also includes the ID number of the Notified Body which conducted the certification. The image to the right shows an example of a CE mark on a medical device. 0543 is the Notified Body ID, which can be looked up here. Notified bodies are only authorized to certify specific product types, and have those abilities listed on their page.
The China Export Mark
The China Export Mark means that the product was manufactured in China. There is no registration, testing, or auditing required in order to use it. The mark can be used arbitrarily by Chinese manufacturers. Products bearing the China Export mark vary in quality from being acceptable to outright dangerous in their design, and require constant vigilance on the end user’s part to be used safely.
It is common practice for engineers to fully disassemble and inspect products of suspect quality, especially products which operate from AC mains power such as USB chargers, and battery chargers. Many engineers have personal anecdotes of using poorly performing products that appeared to have a CE mark, which turned out to be a China Export mark. One common shared experience is that of power supplies which become dangerously hot, sometimes to the point of melting their own casings.
The Differences
Given the vast difference in the meaning of these symbols, you need to be able to tell them apart. The image at the beginning of this blog (courtesy of wikimedia commons) shows a comparison of the legitimate CE and a similar-looking China Export logo.
The letters are formed identically, however the spacing between the letters is different and differentiates the two marks. A proper CE mark has the E start on the circular profile drawn out by the C character. When purchasing products it is good general practice to look at their certification markings and do research to ensure the product actually complies with the CE regulations it falls under.
A manufacturer of a legitimate CE-marked product should be able to provide a Certificate of Compliance, stating the requirements to which the product conforms. Given how heavily regulated medical devices are, conforming products are generally quite safe. However, there are anecdotes of unethical companies looking to save money by submitting technical documents for review, then upon receiving approval, removing components to save money at the expense of safety or longevity.
Other Marks
There are a number of other regional bodies which test products for various safety and standards compliance. UL provides safety certification, inspection, and training to companies. Having UL certification is not required to sell products in North America, however companies wishing to get liability insurance for their products need to have proper external audits done to qualify. The CSA Group performs product safety testing, and competes with UL in that area. Both organizations belong to the Nationally Recognized Testing Laboratory (NRTL) program. There are a number of testing agencies which are certified to test to certain standards. More information about that can be found here. The Federal Communications Commission (FCC) is a United States government organization. Products which conform to FCC regulations emit electromagnetic interference under specified limits.
Conclusion
There may be a large variation in quality between a legitimate CE-marked product and a look-alike, and it is important to be aware of what you’re buying. Otherwise, it might hurt. in this blog you have learned how to identify differences between the CE vs China Export Mark are explained along with information on meanings, regulations and visual layout of the two marks.
Being able to spot the difference between the two marks doesn’t quite save you from counterfeits, as there are vendors who place fake UL, CE, and other markings on their products. Constant vigilance on the part of engineers and supply chain management is needed to ensure you’re using genuine parts and avoid costly mistakes.
Ashwin Sira is a former StarFish Medical Junior Electrical Engineer. He has a passion for electrical power electronics, robotics, controls, and embedded systems that he incorporates into his work in medical device Product Development. This is his first blog for StarFish Medical. He would enjoy hearing feedback and ideas from readers.
Image: Wikimedia Commons
