I’m often asked how ERP benefits medical device commercialization. Enterprise resource planning (ERP) is a very powerful tool that helps many companies succeed. The medical device industry faces unique challenges from FDA and CFR requirements, regulations, and standards. These challenges mean that ERP can aid medical device commercialization even more than other industries and products.
ERP is:
- a software infrastructure that supports managing and coordinating various company processes and workflows in order to achieve specified goals
- a combination of business management practices and information technology integration to achieve business objectives
Why ERP?
A company that is constantly growing, but using multiple disconnected reporting systems, is like activating the emergency brake system while trying move ahead.
Some might be reluctant to give up old software and practices. I encourage them to think of ERP like “All-Inclusive” tickets. One program gives you everything you need in one place. In fact, the more applications and software a company is managing, means more costs, more room for mistakes and more time consumed. Combined, these items mean lower efficiency.
Technological advantages of ERP
- Increase efficiency
- Replace multiple systems with a single independent system
- Gain information control in a single database
- Integrate supporting systems with business process
- Replace multiple systems with a single independent system
- Optimize inventory
- Global online access
- Improve project reporting
- Business Intelligence (BI) software compatible
- MechWork, SolidNetWork and barcode scanning compatible
- Business Intelligence (BI) software compatible
Operational advantages
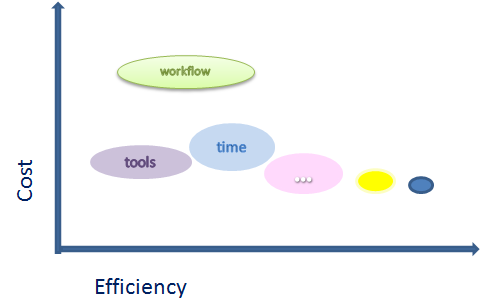
- Reduce operational costs
- Improve workflow and coordination
- Provide a bird’s eye view of overall business operations
- Improve financial management
- Standardize process
- Track project time
- Standardize process
- Simplify integration of new businesses (acquired companies) into current technological infrastructure
- Simplify business planning
HOW?
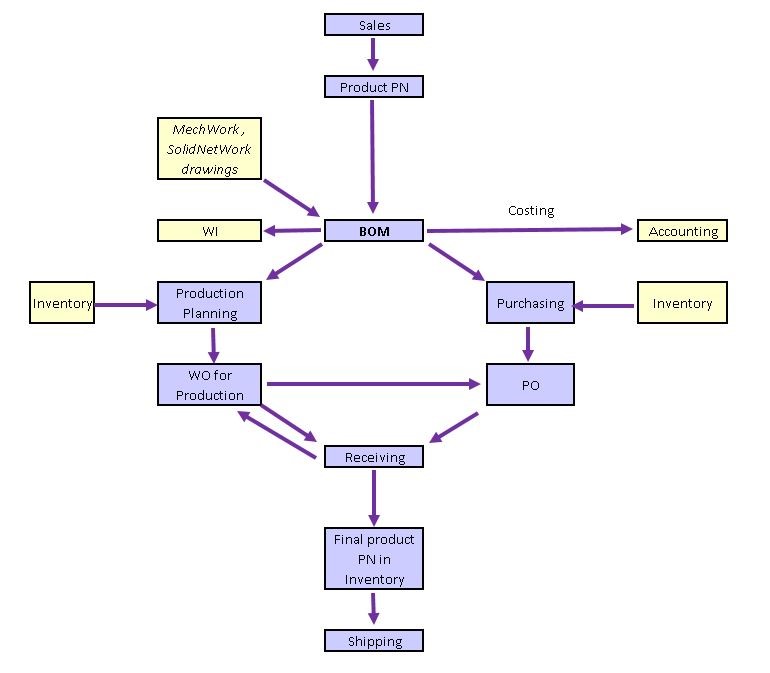
ERP applications are designed to integrate all company processes and operation management into a single database. This database includes modules that manage: sales, product development, engineering, accounting, payrole, inventory, purchasing, manufacturing, logistics, personnel, CRM, quality control QA reports. An ERP application connects all of the database modules and provides all of the information you need just one click away.
Here is how it works. After a sale is closed, a Part Number (PN) is created for the future product and associated with the Sales Number. This new PN will generate demand for Product Development to develop the Bill of Materials (BOM). The new BOM will generate demand for Work Orders and Purchase Orders. The Inventory database will have real-time input on these actions. All of these activities and resulting data are linked together beginning with the client’s Sales Order through to the final product being released back to the client.
ERP and Medical Devices
As noted at the beginning of this blog, an ERP is particularly useful when dealing with the unique challenges the Medical Device organizations must address through the following benefits:
- Consistency and efficiency provided by a single ERP database
- Lots, parts and components traceability through product identification and serialization required for warranty, service and recalls, and regulatory compliance
- Document and quality control – through consistent device history records, Engineering Change Requests, (ECRs) and Engineering Change Order (ECOs)
- No errors through corrective and preventive actions (CAPA)
- Visibility and control over the entire process.
I hope this blog helps you understand how ERP benefits medical device commercialization address its unique challenges from FDA and CFR requirements, regulations, and standards. I would be happy to hear from readers to share advice and experiences.
Laura Gouzecky is a Project Coordinator at StarFish Medical. Laura is a Mechanical Engineer who has project management, Lean Six Sigma and purchasing training. This is her first blog For StarFish.
Images: StarFish Medical
