
Irvine – StarFish Medical (formerly Omnica)
Medical Device Design & Engineering – Irvine
Advancing U.S. MedTech innovation from Southern California
StarFish Medical’s Irvine office serves as our U.S. development hub and the newest addition to our North American footprint.
Located in the heart of Orange County’s MedTech corridor, this site supports U.S.-based companies with local expertise and seamless access to StarFish’s national team of 100+ engineers, regulatory experts, and commercialization strategists.
Formerly known as Omnica Corporation, a respected product development firm in Southern California, the Irvine team brings over 40 years of experience in industrial design, engineering, and device innovation. StarFish Medical acquired Omnica in November 2024, integrating its legacy of technical excellence and creativity into a full-service medical device development offering.
Full-Service Medical Device Development in Irvine
Our Irvine-based engineers, designers, and program managers support every phase of medical device innovation — from early concept through to manufacturing handoff:

Medical Device Design & Systems Engineering
Multidisciplinary integration of hardware, firmware, software, and optical systems, developed to meet regulatory, clinical, and business requirements.

Human Factors Engineering & Usability Testing
Embedded human factors support ensures user-centered design and compliance with FDA and international standards.

Design for Manufacturing (DFM)
Our team engineers for manufacturability from the start, supporting component selection, cost analysis, and vendor alignment for scalable production.
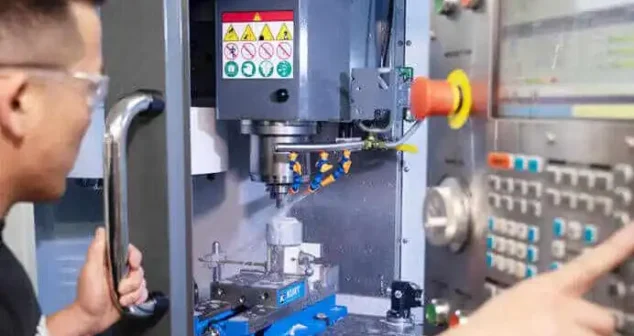
Medical Device Manufacturing Support
We facilitate pilot builds, generate comprehensive documentation, and prepare devices for seamless transfer to contract manufacturing organizations (CMOs).

Regulatory Strategy & Quality Systems
FDA and ISO 13485-aligned development, design controls, and submission support integrated throughout the product lifecycle.

Commercialization & Business Alignment
We support venture-backed startups and growth-stage companies in aligning technical execution with investment, market entry, and partnership strategies.

Building on Omnica’s Legacy of Innovation
For more than four decades, Omnica was known throughout Southern California for its deep technical talent and creativity in product design and engineering. That legacy continues under StarFish Medical, enhanced by a broader infrastructure, expanded regulatory resources, and full commercialization capabilities.
Our Irvine office retains the collaborative culture, design excellence, and hands-on engineering leadership that made Omnica a trusted partner to medtech companies across the U.S.
Areas of Expertise
Our team supports a wide range of devices and technologies:
- Wearables & digital therapeutics
- Diagnostics & point-of-care testing
- Surgical & therapeutic systems
- Optics, imaging, and electro-mechanical platforms
- Minimally invasive and Class III medical devices
Whether you’re refining a concept, building a regulatory-ready prototype, or preparing for clinical trials and manufacturing, we provide technically rigorous, scalable solutions.


Connected to Southern California’s MedTech Community
We actively engage with the local ecosystem through partnerships with:
- Octane – accelerating growth and investment in high-impact medtech ventures
- DeviceAlliance – connecting Orange County’s medical device professionals and fostering leadership
Frequently Asked Questions
Did StarFish Medical acquire Omnica?
Yes. StarFish acquired Omnica Corporation in November 2024. The team and site continue to operate in Irvine as part of StarFish Medical, maintaining the same commitment to quality, innovation, and hands-on engineering.
What types of devices does the Irvine team develop?
We support medical devices across a wide spectrum, including surgical tools, diagnostics, wearables, imaging platforms, and energy-based therapeutics.
Do you provide medical device manufacturing in Irvine?
We support all aspects of pilot builds, documentation, and design transfer to trusted contract manufacturing partners.
Can you support FDA 510(k), De Novo, and PMA submissions?
Yes. Our integrated regulatory team and QMS framework support U.S. submissions and design controls from early development onward.
Let’s Build the Future of MedTech in Irvine
Looking for a strategic partner in medical device design, engineering, or manufacturing?
Related Resources

Nick and Nigel walk through how sterile disposables are processed and verified before they reach the field.

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.

The sandwich ELISA assay is one of the most common ELISA formats used in diagnostics. Nick and Nigel walk through the method step by step using simple visuals and plain language.