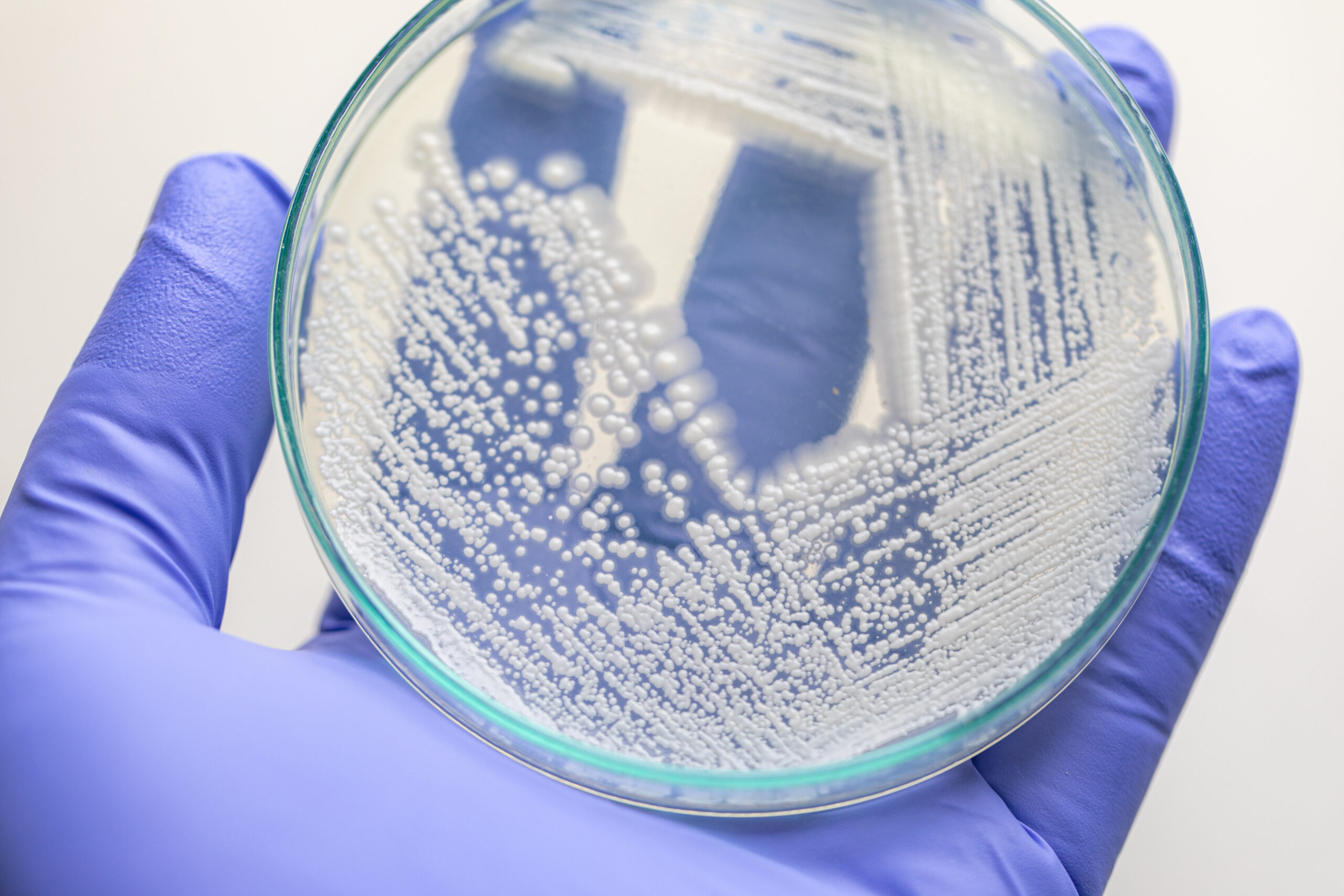
The article, Assessing Mold Growth Severity on Various Textiles: Comparative Analysis of Inoculum Densities and Fungal Strains, explores how different textiles respond to fungal exposure under real-world shipping conditions—findings that have important implications for medical device materials and packaging.
Nick Allan, Bio Services Manager at StarFish Medical, co-authored the study published in Sustainable Microbiology examining mold contamination risks on textiles during overseas shipping.
Mold contamination in transit is a significant concern for industries that rely on sterile, high-integrity materials, including medical device manufacturers. This study evaluates the susceptibility of natural and synthetic textiles to mold growth, testing six fungal strains—including Aspergillus niger, Penicillium citrinum, and Stachybotrys chartarum—at varying inoculum densities. Using simulated shipping conditions, the research identifies the minimum contamination thresholds required for visible mold growth on various textiles.
Findings indicate that natural fibers, particularly cotton and suede, are highly vulnerable to mold growth, even at lower spore densities. Leather exhibits moderate resistance, likely due to antimicrobial agents used in the tanning process. In contrast, synthetic materials such as polyester, imitation suede, and polyurethane demonstrate significantly higher resistance to fungal contamination. The study also highlights the role of environmental factors such as humidity, material composition, and surface treatments in determining mold proliferation.
These insights are particularly relevant to medical device engineering, where mold contamination can compromise sterility, impact the performance of textile-based components (such as wound dressings, surgical drapes, and wearable medical devices), and introduce regulatory concerns for shipping and storage. Understanding mold susceptibility and preventive strategies can inform the selection of medical-grade materials, improve packaging design, and enhance quality control measures for devices that incorporate textiles or rely on sterile shipping conditions.
This study also aligns with the UN Sustainable Development Goals (SDG 3: Good Health and Well-Being and SDG 12: Responsible Consumption and Production) by promoting safer consumer products and sustainable manufacturing practices.
About StarFish Medical
StarFish Medical provides award-winning design, development, commercialization, and flexible manufacturing outsourcing services —100% dedicated to the medical device and life science marketplace. StarFish Medical partners with innovative companies to create and manufacture breakthrough products for a full range of medical specialty areas including: Digital Health, Cardiovascular, Neurology, Urology, Gastroenterology, Otology, Ophthalmology, and In-Vitro Diagnostics.
StarFish Medical’s technical expertise includes electronics, mechanical, software/firmware systems engineering, in addition to industrial design and human factors. Regulatory Affairs (RA) and Quality Assurance (QA) consultants at StarFish Medical provide regulatory assistance for FDA, CE Mark and Health Canada submissions. Services include QA support for setting up QMS for start-up companies with implementation at the client’s site, and assisting with ISO 13485 certification audits..
Empowering Medtech Innovation®. www.starfishmedical.com
Contact Patrick Dean, Director of Marketing, for media inquiries.