
Medical device design & development
Empowering MedTech innovation to create breakthrough products
that improve health and save lives
At StarFish Medical, we blend deep technical expertise, regulatory insight, and user-centered design to bring MedTech innovation to life. From startups to global enterprises, we turn visionary ideas into breakthrough products that improve health and save lives.
25+
Years
200+
Professionals
1000+
Programs
Our Services
Working with StarFish
From early-stage startups to global MedTech leaders, we help companies solve complex challenges and turn bold ideas into market-ready products.

Meet ELLA
Introducing ELLA, an AI-powered tool designed to transform how medical device companies navigate regulatory compliance.

Latest Resources
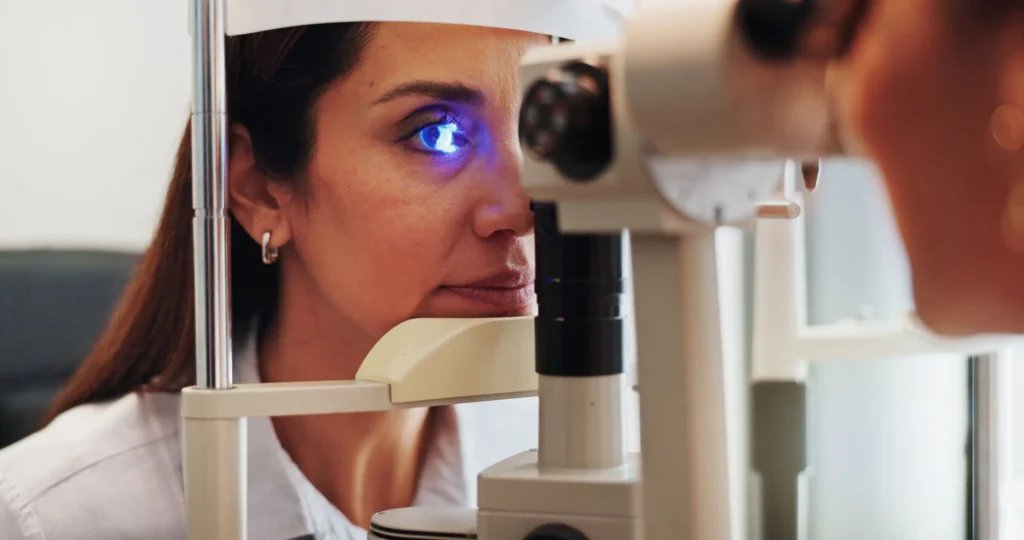
I was recently looking through the OPTICA trade journal Optics and Photonics News – specifically its summary of “Optics in 2025.” A few highlights were of particular interest to me in terms of their potential applicability to future medical devices.
Ariana Wilson and Mark Drlik explore how bias can enter the development process and why engineers and manufacturers must actively work to prevent it.

Did you know that 5-8% of total national carbon footprints come from the healthcare sector? Much of this (around 80%) is general waste – such as from office work – and the rest (~20%) requires special handling due to its dangerous nature.

Electrocardiography (ECG) remains the gold standard for non-invasive cardiac assessment, providing a vital window into the heart’s electrical health. By recording electrical impulses through surface electrodes, clinicians can identify life-critical conditions such as arrhythmias, myocardial infarctions, and conduction abnormalities.
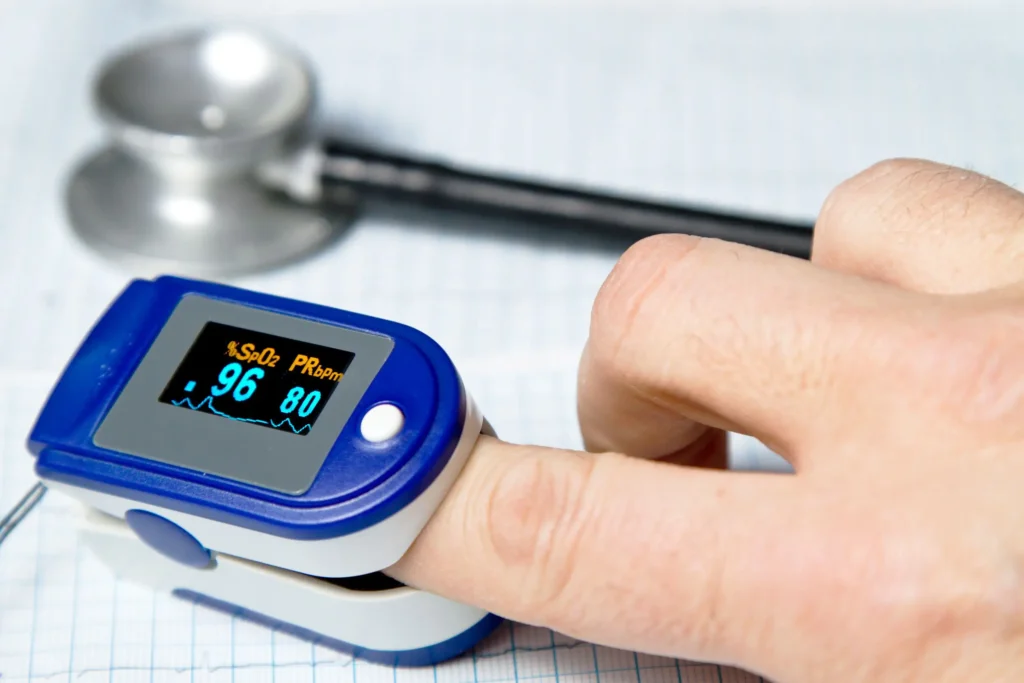
Wearable medical and wellness devices are increasingly commonplace in the healthcare field. For example, devices such as smartwatches incorporate sensors to provide continuous health tracking data to their users.

Nick and Nigel explore how much bacteria can exist on devices and why it matters. They explain that bacteria are everywhere.
Sectors We Serve
Axolotl BrainPrint Microsphere Generator

Aurora DNA Sample Preparation Device
Join over 6000 medical device professionals who receive our engineering, regulatory and commercialization insights and tips every month.