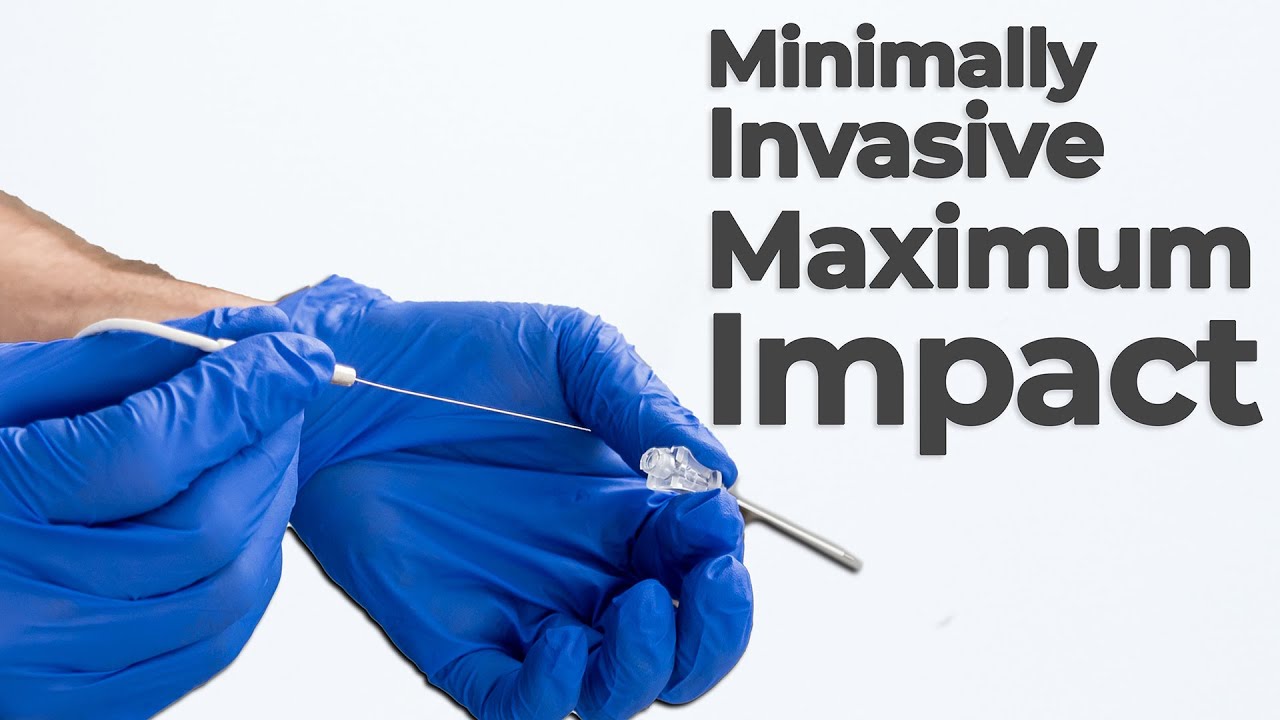
Bio Break: How Ablation Technology Is Transforming Surgery
In this episode of Bio Break, Nick Allan and Joris van der Heijden dive into one of the most impactful trends in modern medtech: minimally invasive surgery. Ablation technology plays a crucial role as hospitals and healthcare providers aim to reduce patient recovery times and overall system strain. Technologies like ablation have emerged as a powerful tool for treating conditions once requiring intensive surgeries.
Joris explains how ablation enables tissue removal without the need for large incisions. Whether it’s chemical, electrical, or thermal ablation, these methods allow clinicians to precisely target and destroy problematic tissues. The result? Less time in the hospital, faster healing, and a more comfortable experience for patients. Nick shares that while he once expected such treatments to be reserved for serious operations, technologies like ablation are now being used in areas like tumor removal, pain management, and cardiac arrhythmia treatment.
The two highlight how a flexible catheter can navigate the body to reach affected tissue sites without opening up the patient. From chronic back pain to small tumor removal, ablation technology delivers precise, targeted therapy with far fewer complications than traditional surgical procedures.
But with all its benefits, ablation isn’t without its limitations. Because the body isn’t fully opened up, visibility is limited—requiring more sophisticated imaging technologies to guide the process. As Joris points out, this often restricts the procedure to larger clinical centers, but the benefits to both patients and the healthcare system are still profound.
Whether you’re a developer of medical technologies, a healthcare provider, or simply curious about how next-gen surgical tools are reshaping patient care, this episode covers the significance of ablation technology and offers an informative and approachable look at a high-impact innovation.
Related Resources
I was recently looking through the OPTICA trade journal Optics and Photonics News – specifically its summary of “Optics in 2025.” A few highlights were of particular interest to me in terms of their potential applicability to future medical devices.
Ariana Wilson and Mark Drlik explore how bias can enter the development process and why engineers and manufacturers must actively work to prevent it.

Did you know that 5-8% of total national carbon footprints come from the healthcare sector? Much of this (around 80%) is general waste – such as from office work – and the rest (~20%) requires special handling due to its dangerous nature.

Electrocardiography (ECG) remains the gold standard for non-invasive cardiac assessment, providing a vital window into the heart’s electrical health. By recording electrical impulses through surface electrodes, clinicians can identify life-critical conditions such as arrhythmias, myocardial infarctions, and conduction abnormalities.