
Bio Break: The Story of PCR and Taq Polymerase
In this episode of Bio Break, Joris van der Heijden and Nick Allan continue their exploration of nature-inspired innovations, focusing on one of the most transformative breakthroughs in molecular biology: the polymerase chain reaction (PCR) and its key component, Taq polymerase.
Joris recounts the discovery of Taq polymerase, an enzyme found in Thermus aquaticus, a heat-resistant bacterium discovered in the hot springs of Yellowstone National Park. This enzyme’s ability to withstand extreme temperatures made it foundational to PCR, a technique that revolutionized molecular biology, genetic testing, and forensic science. The discovery was a turning point, enabling scientists to replicate DNA at high temperatures without degrading the enzyme—a feat that has had a profound impact on laboratory research worldwide.
The hosts discuss the pivotal moments in PCR’s history, including its invention in 1983 by Kary Mullis, and its vast applications today, such as:
- Infectious disease testing
- Genetic screening and diagnostics
- Forensic science and criminal investigations
They also delve into advancements in enzyme engineering, explaining how human ingenuity has improved the functionality of natural enzymes like Taq polymerase. Examples include the development of hot-start polymerases for precise DNA replication and proofreading enzymes for increased accuracy. Recent breakthroughs, like isothermal replication, eliminate the need for thermal cycling, simplifying workflows for medical devices.
This episode highlights how millions of years of evolution have provided tools that humanity can refine for critical applications. The story of Taq polymerase exemplifies the powerful synergy between nature and science, showcasing how discoveries rooted in the natural world can drive innovation in healthcare and beyond.
Whether you’re a scientist, engineer, or simply curious about the intersection of biology and technology, this episode offers a fascinating glimpse into how nature-inspired solutions are shaping the future of medical devices.
The Story of PCR and Taq Polymerase
For Startups or Founders developing a device that incorporates novel technology or is like nothing else, the FDA breakthrough medical device program may be the best regulatory option. Learn more.
Related Resources

A structured, well-documented design review process is a critical component of successful product development, particularly in the medical device industry.

In medical device development, we deal with complex projects that span multiple disciplines, timelines, and regulatory gates. It’s a constant balance between moving fast enough to innovate, but slow enough to stay compliant.
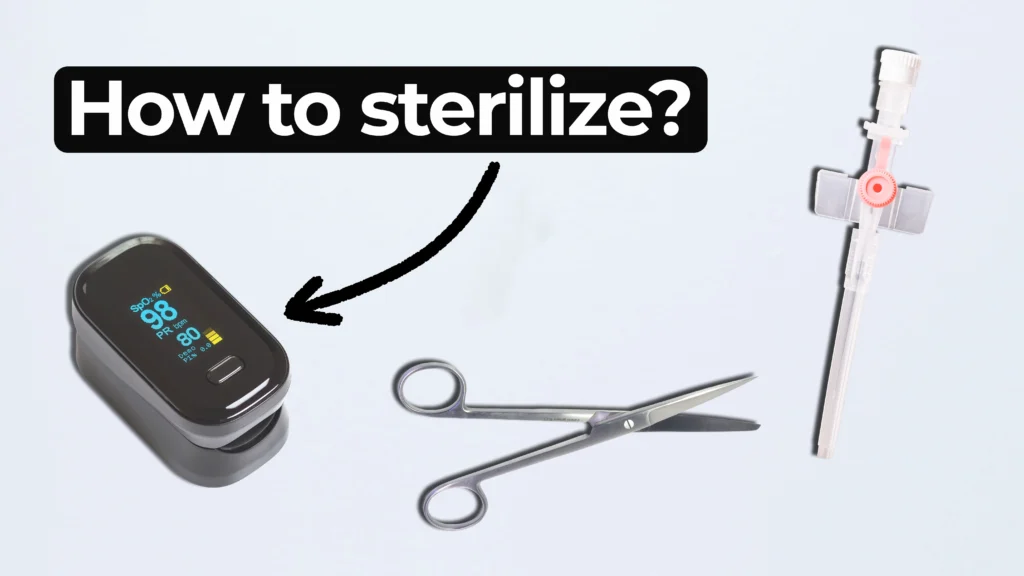
Ariana and Mark walk through FDA-approved options and explain how to select the right one for your product. From metals to plastics and electronics, not all devices can handle the same process.
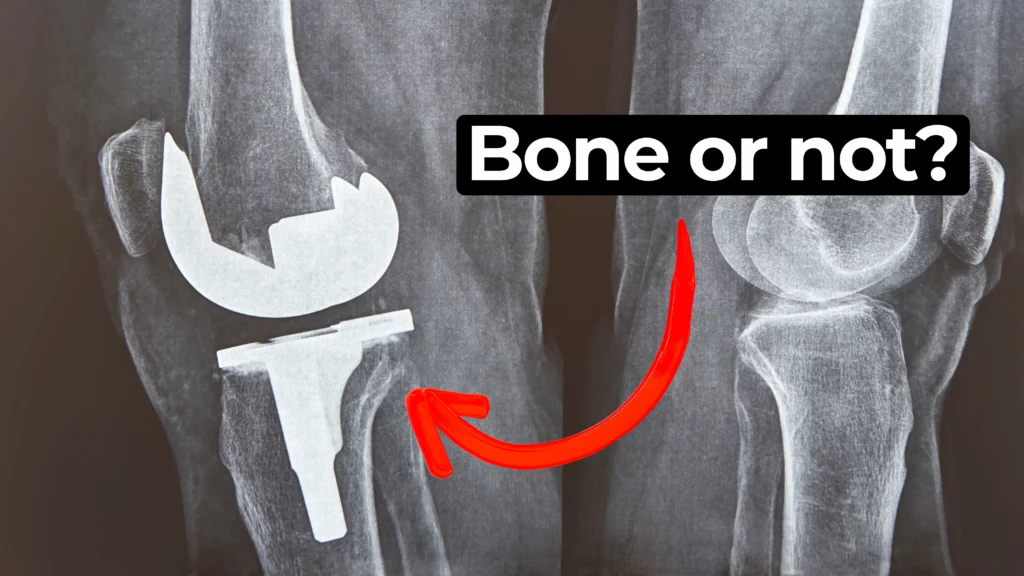
In this episode of MedDevice by Design, Ariana and Mark dive into the biomechanics and materials science behind osseointegration for implants.