Computational Modelling and Simulation in Medical Device Development
Abstract
In a sophisticated world of ever increasing complexity, we need our tools to evolve alongside us and assist in complex decision making, allowing us to understand the consequences of choices ahead. Computational Modelling and Simulation (CM&S) is emerging as an essential tool in building evidence for medical device development, providing increased confidence in technical feasibility during development and providing evidence of safety and efficacy for market clearance submissions to regulatory bodies. This paper offers a broad overview of CM&S and insights into what they are; why, where, and how CM&S models are applied to medical device development; and their future contributions to the industry. This paper will demonstrate the clear benefits offered by CM&S to the industry by considering all of its merits to increasing quality in development, scaling studies, reducing animal harm, and decreasing time to market, all to the benefit of patients and companies involved in medical device development.
Intro
Design and development in the medical device industry is continually modernizing, with Computational Modelling and Simulation (CM&S) playing a growing role in the sector. Its role is expected to increase further, with industry and regulatory agencies promoting its use across more domains. In silico (i.e., computer simulation) studies are being used as an integral part of medical device development to virtually assess and test devices and their use scenarios. CM&S allows more comprehensive de-risking during development and is also used as evidence in regulatory submissions, especially where it has distinct advantages over physical testing. This whitepaper explores how medical device development can take advantage of CM&S to increase value for programs.
What is Computational Modelling and Simulation?
Definition
CM&S is the use of computers and their programs to study complex systems. Using algorithmic or mechanistic approaches, it is used widely in diverse fields. These fields not only span STEM: physics, engineering, chemistry, mathematics, medicine, and biology; but also include a broader range of disciplines, including economics, psychology, cognitive science, and computer science. Once computational models are established, they are used to predict behaviours in the real world and run simulations. They can be validated with real world experiments or analytical solutions to increase confidence in a base model, and then leveraged to explore parametric space for design exploration and risk assessments. This is particularly useful in medical device design, where CM&S can be used as a tool to better inform and expedite design exploration in early programs and risk assessments for those seeking regulatory approvals.
Computational Model versus Simulation
It’s important to differentiate the term Computational Model from Simulation, due to them often being used in combination with one another, but sometimes on their own (e.g., “a Computational Model” or “a Simulation”). Computational Models are the algorithms and equations used to capture the behaviour of the system being modelled; by contrast, computer simulations are the running of programs that contain equations or algorithms, and their results. Therefore, a simulation is the running of the model or its output, and a computational model is the code that is built and used in a program to run these specific simulations.
Why Use Computational Modelling?
Medical device development is a costly activity, involving large investments of capital and time. This is partly due to the high bar set by regulatory agencies to prove safety and efficacy before bringing products to market. Another is the novel state of many of these technologies, sometimes being new inventions in the field and therefore unproven. Engineers use an array of methods to provide evidence of a device’s technological readiness, efficacy, and safety. Building and testing physical prototypes is a large part of this process. It’s also a resource intensive endeavor, involving in-depth design and development efforts by an array of highly skilled personnel. The traditional approach to medical device development relies heavily on evidence gained from bench experiments, animal testing and clinical trials. These proof-of-concept activities – often large in scale extending over a long duration – are used to provide evidence for safety and efficacy of the device, enabling regulatory body clearance for varied markets. Due to these factors, the time to market for medical devices is relatively long compared to other product development streams.
CM&S provides an alternative to traditional methods of testing and offers compelling areas of utility, such as scaling tests through parametric studies. It also enables product development to be expedited as designs alternatives can be assessed and filtered rapidly at early stages with low capital costs, allowing the leading designs to be tested virtually, prior to prototyping. CM&S has the capability to create many iterations of virtual designs and scenarios in rapid succession before engineers build the first physical prototype. It can lend itself to a “fail fast” approach, allowing for assessments to bring failure modes to light along with other means of design improvement and optimization. This serves to reduce timelines and address risks of development in silico. Additionally, by reducing animal studies and trials, CM&S can reduce the suffering and unnecessary death of animals. When used judiciously, CM&S decreases time to market and provides an impetus for development of disruptive and innovative technologies.
Market Projections for CM&S-Enabled Development
The global medical device and pharma market is expected to exceed 2,150 billion dollars by the end of 2025. With medical device development increasingly enabled by modelling and simulation, in silico enabled medical device development is projected to exceed 109 billion dollars by 2025. Figure 1 provides an overview of market capitalization for total market vs. in silico enabled portions.
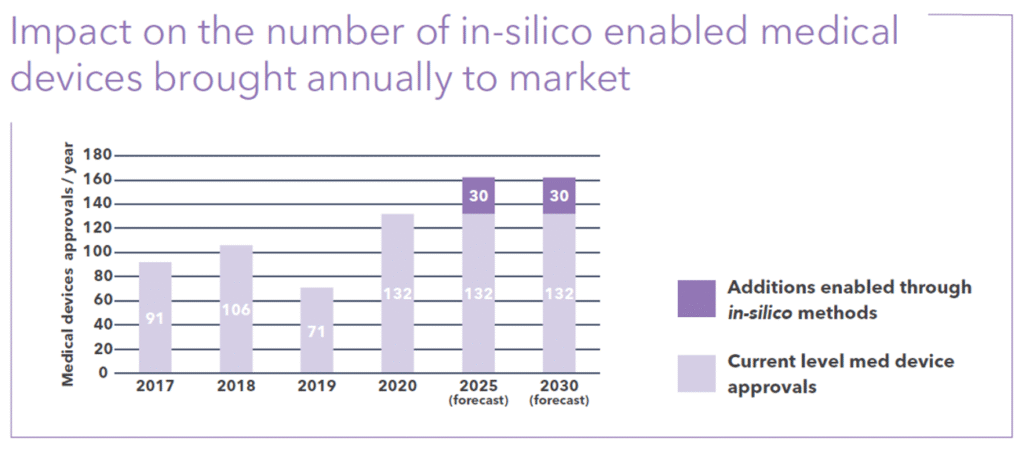
By 2025, in silico methods are expected to bring an additional 30 new medical devices to market every year. Figure 2 shows these projections, providing the impetus to increase investment in CM&S tools and development using them.
Greenlighting from Regulatory Agencies
Medical devices exist in a heavily regulated field and professionals involved in their development must abide by stringent guidelines for evidence that provides confidence in a product’s safety. The increased application of CM&S to medical device development includes activities such as: design initiation, prototyping, redesign, optimization, and risk/failure analysis. Its use is also projected to increase in the near future [2]. Regulatory bodies are helping initiate the shift, as they recognize the potential benefits for society and the market.
The FDA identified in silico studies as a key area of opportunity and made it one of its priority areas in their 2018 strategic policy roadmap. The vision of regulatory bodies is to encourage and normalize the use of CM&S in medical device development. In pursuit of this, FDA also included CM&S in its strategic policy roadmap, the Medical Innovation Access Plan [3]. It includes their desire to “cultivate new policies and guidance for product regulation in key areas of novel medical science, with the goal of creating pathways that allow beneficial new technologies to efficiently reach patients while strengthening FDA’s gold standard for product safety and effectiveness” through “[Advancing] the use of in silico techniques to develop novel methods for creating models of virtual patient outcomes and modernizing FDA’s evaluation of patient benefit and risk” [3].
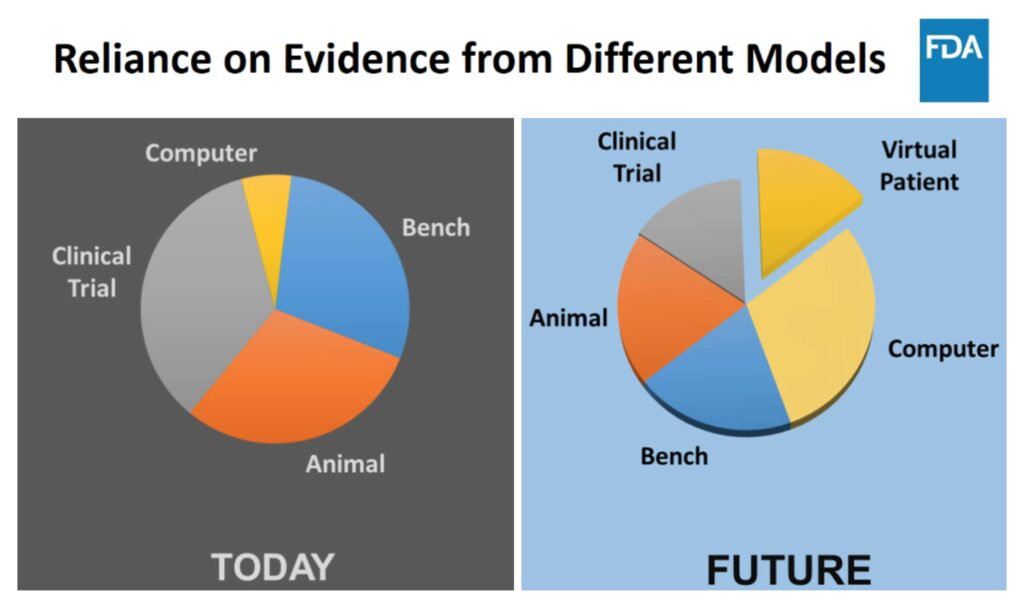
While CM&S is currently 15% of gathered evidence for medical device submissions, it is projected to increase to the largest proportion of evidence at nearly 50% in the near future (inclusive of computational and virtual patient models). The FDA provided this projection with context in a 2018 summary by Tina Morrison Ph.D., their director for the office of regulatory science and innovation and chair of FDA Modeling and Simulation Working Group. Their projection is shown in Figure 3, with the stated intent that using CM&S and Virtual Patients will provide more meaningful and targeting evidence of the safety and efficacy of medical devices as part of their submissions.
Insights from Regulatory Agency Data
The FDA and Health Canada maintain strict oversight by collecting and analyzing data throughout the regulatory process and subsequent post-market surveillance. These bodies ensure that risk to the patient is minimized in products cleared for market use, and categorize medical devices based on the risk they pose to the patient, publishing relevant statistical data for the general public. This data provides insight into where failures have occurred in the past, allowing foresight in new medical device development.
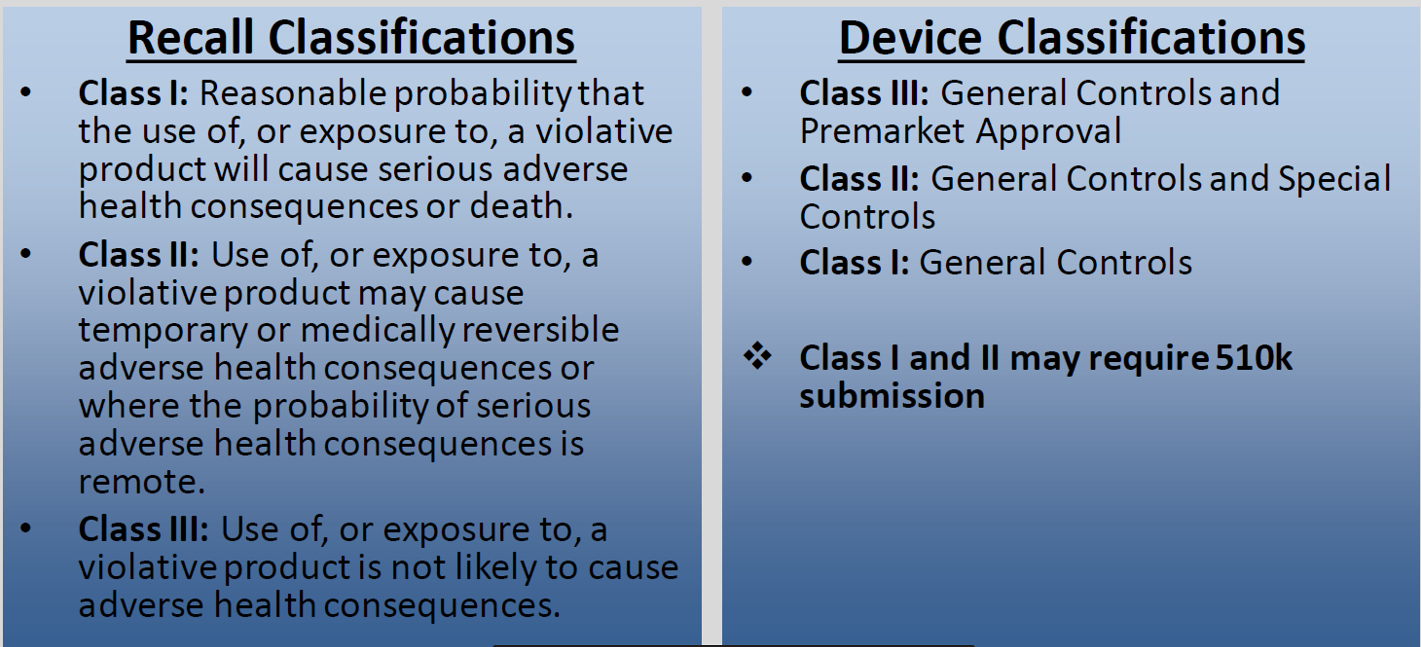
The FDA maintains the record of recall data in USA. Note that the FDA classifies the recalls based on the probability of causing harm or adverse effects. Recalls with the highest probability of causing harm to patients are categorized as Class I whereas device recalls posing the lowest probability of harm are classified as Class III.
Using published recall data from the FDA, pitfalls can be avoided during development by learning from others’ mistakes. Class I recall data between Jan 2016 to Dec 2018 shows that device design is the biggest cause of recalls (see Figure 5). This is revealing as it is a key area of control during device development stage, where CM&S can be a crucial tool for assessment early in the development process.
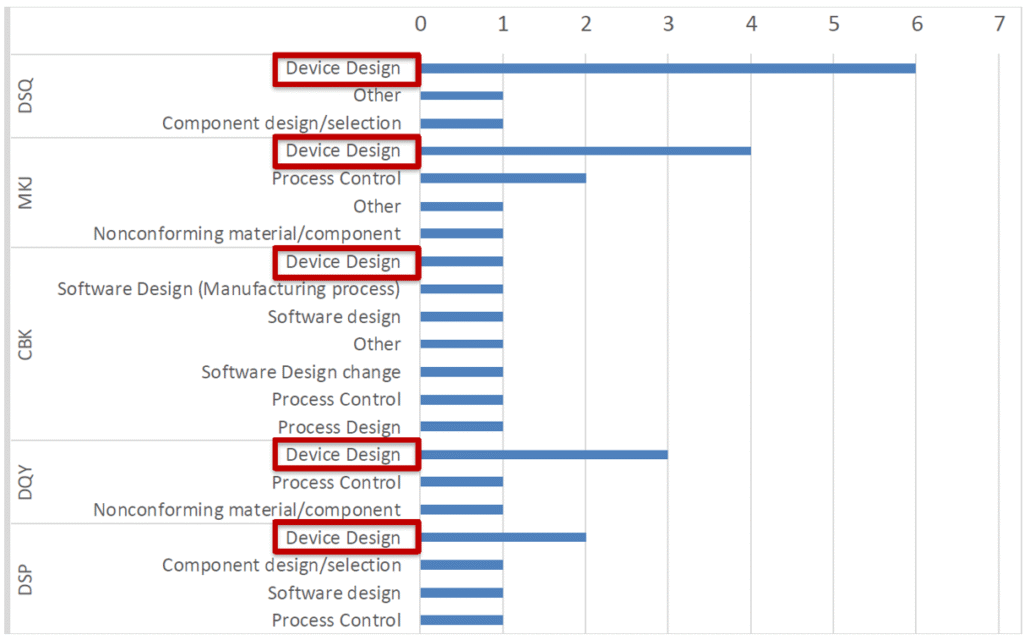
For class II devices, device design is the second major cause of recalls after process control (see Figure 6). This again highlights the need to assess devices early in their development to mitigate risks of failure in the field. For these scaled and parametric studies, CM&S is an indispensable tool.
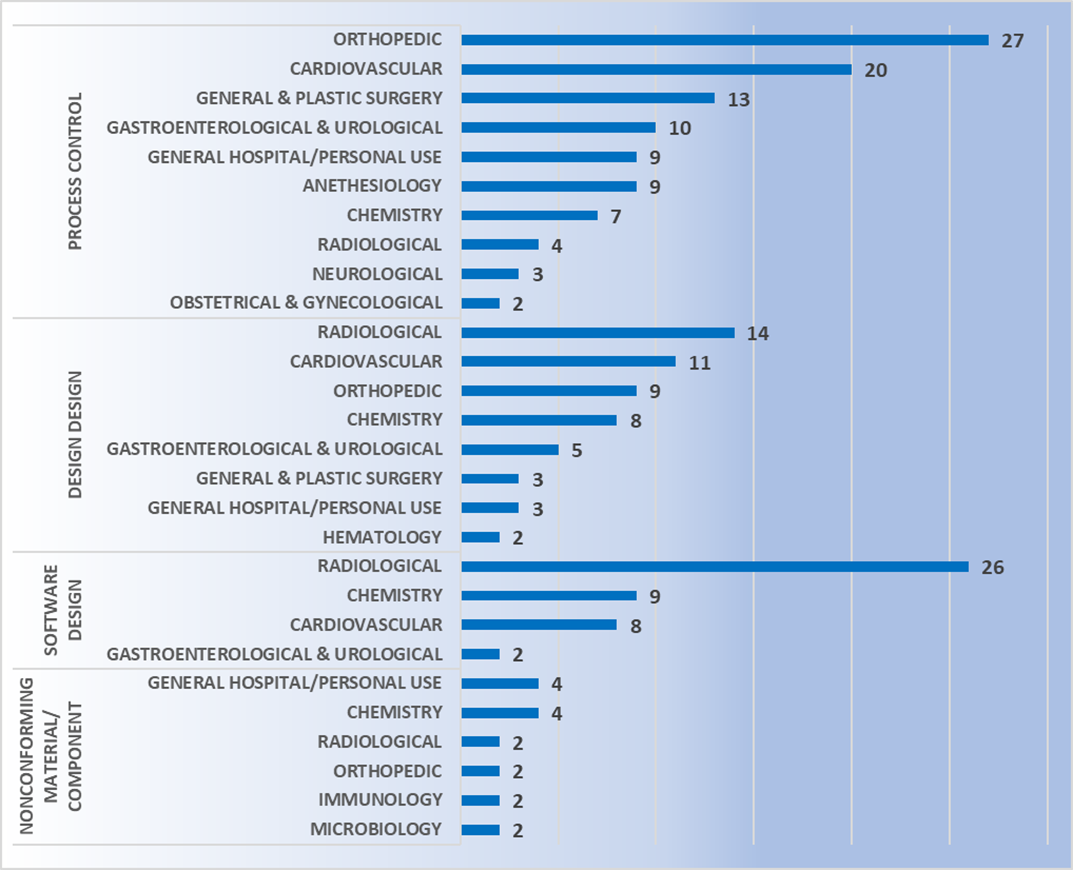
All the data indicates that device design should be scrutinized greatly before product release, especially earlier in design when costs to make changes are lower, as highlighted in Figure 10. CM&S has huge potential to add value to the medical device development process through risk reduction.
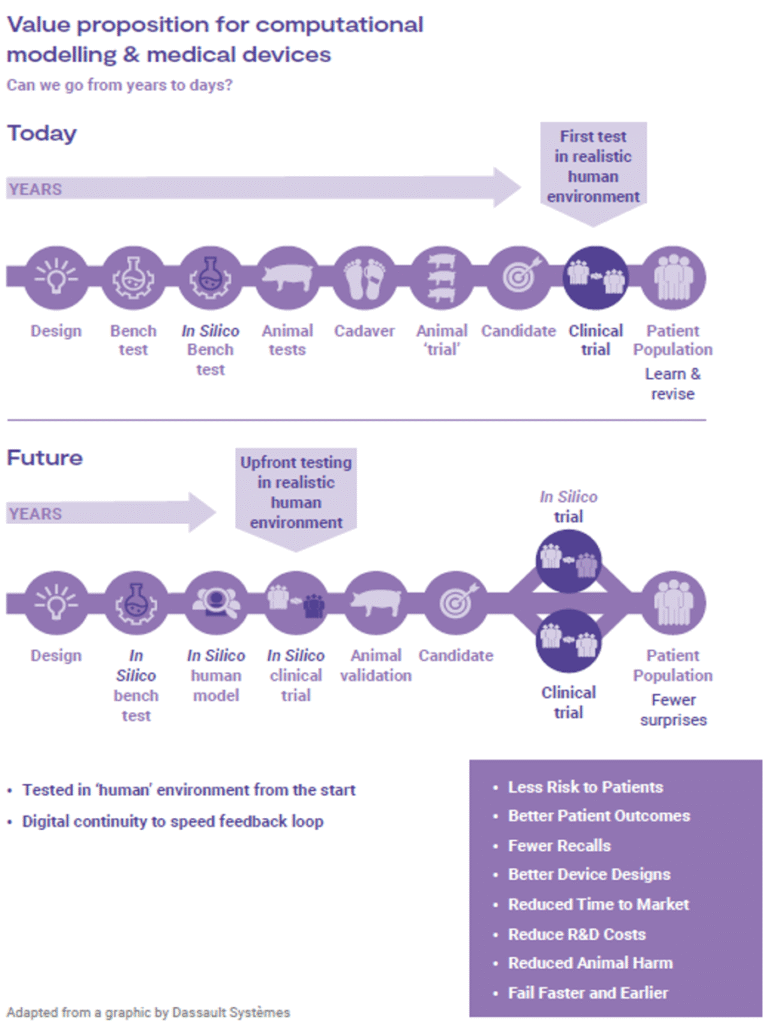
Device design, being one of the biggest risk factors for post market issues, can be further assessed through in silico simulations. CM&S provides necessary flexibility to test an array of design embodiments and parameters to reduce risk by identifying failure modes, testing under extreme conditions, and justifying worst case scenarios. An example is invasive devices: aspects of their validation regime can be tested in silico without exposing patients to unknown risks through clinical trials. Implementing virtual patient models as part of investigations can reveal device behavior and predict associated risks. Figure 7 highlights the ways in which in silico methods can reduce overall timelines, while reducing risk to clinical trial participants, animals, and the general population.
Ethical Considerations: Less Animal Testing
Recently, the Food and Drug Administration (FDA) has promoted and allowed for the use of tests other than animal testing for non-clinical submission evidence. This has sparked an industry interest to shift away from unnecessary animal testing. CM&S provides a great opportunity to de-risk technology and test pre-clinical assessments, helping reduce the amount of animal testing in the development process.
Where and How Models are Used
Using CM&S for de-risking and testing
Medical device development involves many tests that seek to de-risk technological embodiments by increasing confidence in their safety and efficacy. One of the primary concerns for test planning is to meet the IEC 60601 series of standards, which form the cornerstone of safety and performance assessment for medical electrical equipment. Once compliance with these rigorous standards is met, a device developer can be assured of the reliability and safety of medical devices and can mark off a crucial step in their development and validation. Traditionally, physical testing has been the go-to method, but it comes with challenges including being time-consuming, resource-intensive, and costly. In recent years, CM&S has emerged as a potent alternative, offering an array of benefits.
Applicability for Use
CM&S adoption as a preferred approach should not replace all physical testing, but it provides opportunities to align with the technology’s best areas of use. Figure 9 provides an example of how different test modes and trials are best employed for vascular surgery devices. Of note, assessments of use over long periods of time are an ideal area for CM&S, as is adaptability for patient specificity due to the parametric scalability of computational models. It has a high degree of experimental control, due to it being entirely in the purview of the tester, while providing fully assessable outputs. Low costs and ease of assessing a range of parameters are also compelling aspects for CM&S.
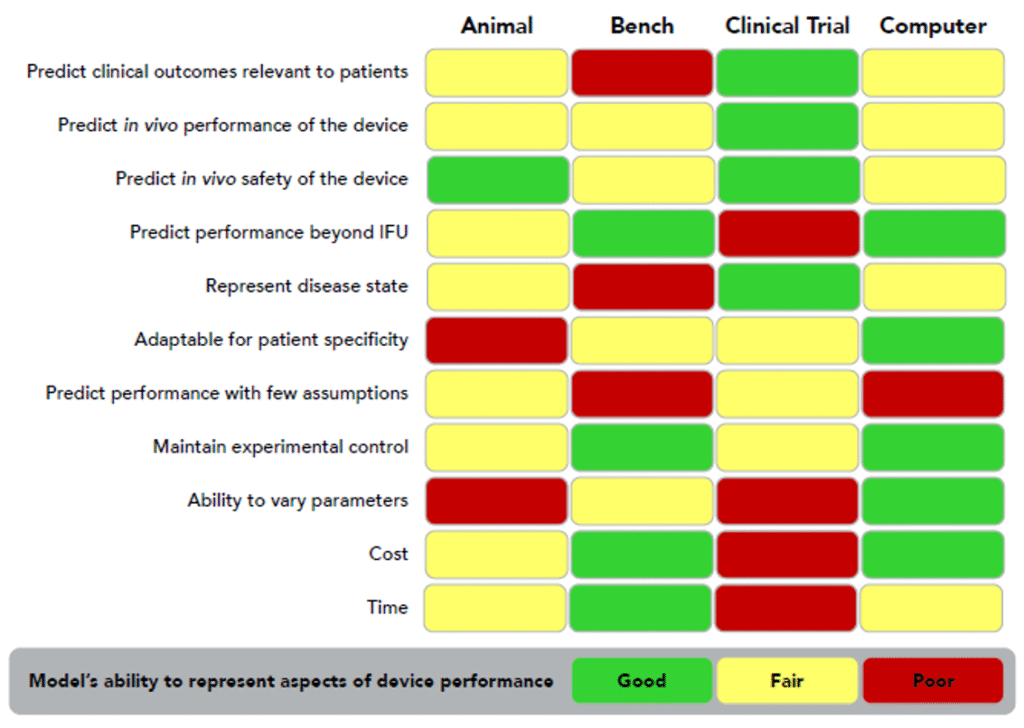
For example, the efficacy of a coronary artery bypass can be fully visualized and parameterized through a computational model with local effects of fluid-solid interactions, while most bench testing offers limited outputs that are holistic or qualitative in nature. Another example is evaluating the performance of a heart valve upon implanting into a virtual patient model to pre-emptively assess any risks associated with the implantation. A final example is a coronary stent, which can be stepwise deployed and assessed for stress in silico, followed by a fatigue assessment informing whether the stent will fail under physiological conditions over its expected lifetime.
Predictive Analysis
CM&S allows for the creation of virtual prototypes of medical devices, which can then be subjected to various simulated tests (such as those specified in ISO 60601). Examples include impact testing – height-based drops for handheld devices and wall impacts for cart-based devices. By employing advanced algorithms and numerical techniques through analysis software (e.g., Ansys) predictions can be made how a device will behave under different conditions. This enables engineers and medical device professionals to identify compliance issues early in the development process, resolve them appropriately, and reduce costs in the long run having brought forward a more robust and reliable design.
Parametric Studies
One of the strengths of CM&S lies in its ability to conduct parametric studies efficiently. By altering input parameters, such as material properties, geometry, and environmental conditions, researchers can quickly evaluate a wide range of scenarios for a given design. This can help when parameters are dependent and difficult to quantify through first principals or tests alone. Also, when nonlinear affects require many tests to characterize responses, parametric studies in silico offer a distinct advantage. Overall, CM&S is particularly valuable for optimizing designs to meet requirements while minimizing iterations and physical prototypes, drastically reducing timelines and material costs.
Failure Mode Analysis
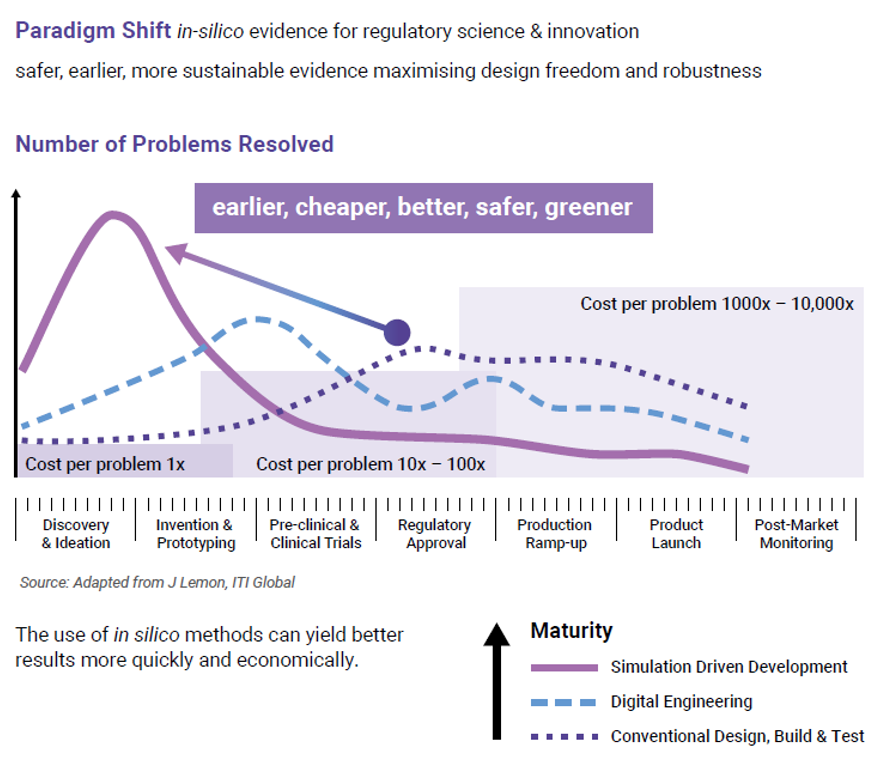
CM&S can simulate various failure modes that may occur during use, especially after risk assessments have identified these. These simulations include mechanical stresses, thermal effects, fluidic issues, and electromagnetic interference. The simulations allow engineers to identify weak points in the design and make improvements before verification testing. This proactive approach enhances safety and reliability while reducing the risk of late-stage design changes. Figure 10 shows how early-stage problems are resolved with lower costs vs. the high-cost of late-stage fixes.
CM&S also provides insight into non-visual damage, which would normally only be detected with high technology inspection (e.g., x-ray or ultrasound) or destructive cross-sectioning. For example, consider an impact test on a solid enclosure with internal components. In silico testing provides full 3D results showcasing internal and non-intuitive damage areas using regular CM&S post-processing. On the other hand, a physical test would take additional planning and subcontracting of specialized imaging and inspection, and still would not yield the resolution (e.g., local and total material damage) provided by CM&S.
Value of CM&S versus Physical Testing
In assessing hypotheses, engineers and medical device professionals should use the most suitable method for the question at hand. Computational modelling offers many benefits over physical testing, and these are growing as models become faster to implement and verification/validation becomes standardized. As such, CM&S approaches are becoming more widely accepted by industry professionals and regulatory bodies, reducing barriers to their use and allowing the benefits to be realized.

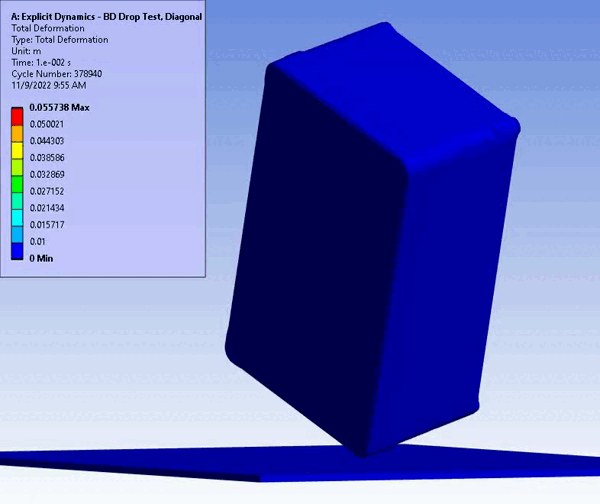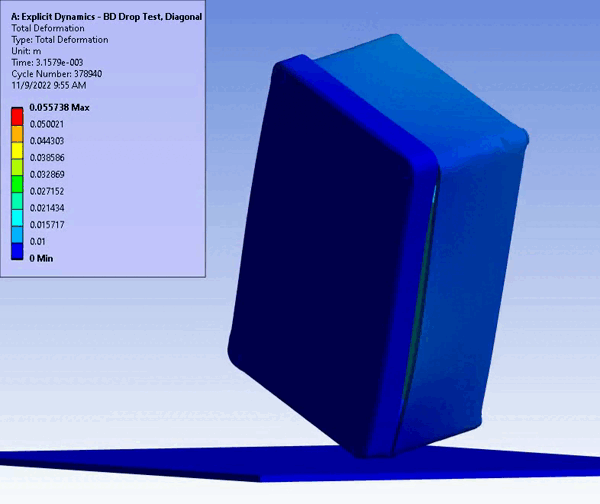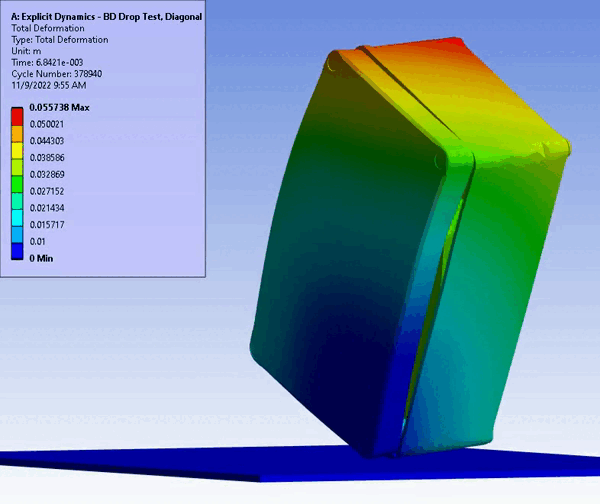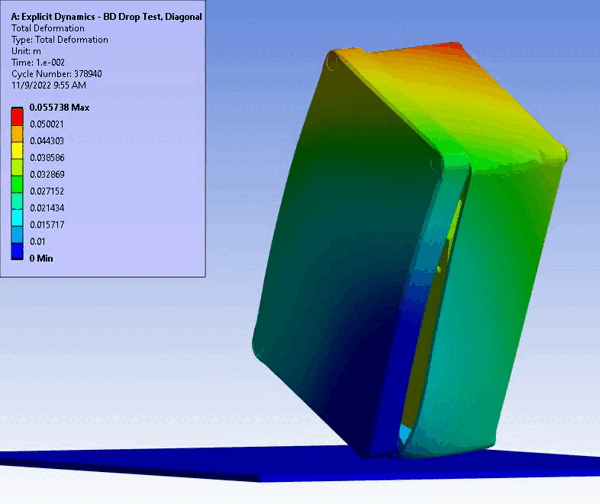
Figure 11 – Drop/Impact Testing – left: physical dropped enclosure, right: in silico dropped enclosure (Source: StarFish Medical)
Resource Efficiency
Physical testing often requires significant resources, including specialized testing facilities, prototypes, and skilled personnel. In addition, once assessed, physical models are often obsolete or damaged due to destructive testing. CM&S both reduces the need for physical prototypes and minimizes the dependency on expensive testing equipment. Though significant investment is required for advanced simulation software and hardware – including having the skills on hand to build, run, and interpret the software and models – the cost is often outweighed by the savings achieved through reduced material and testing expenses, especially where parametric studies are concerned. Figure 11 shows a side-by-side comparison of an enclosure drop test physically and in silico. Note the gradient of deformation in the in silico image – this can be applied to any parameter (e.g., stress) to provide insight into the range at any local region and area. In contrast, the physical model doesn’t allow insight beyond post-inspection, which is limited to visual or destructive tests to get insight on material failures and damage.
Time Savings
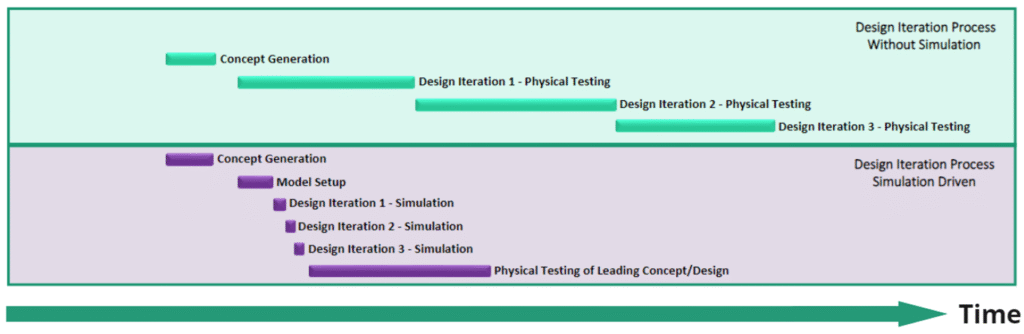
Physical testing involves extensive timelines, including preparation, execution, and analysis phases, which can lead to lengthy development cycles. In contrast, CM&S allows for rapid iterations and quick assessments of design changes, in many cases using the same model for scalable, parametric investigations. This accelerates timelines and is particularly advantageous in fast-paced industries where time-to-market is critical and can provide a competitive edge for companies seeking earlier device release. By the end of investigations, CM&S provides a high value modular computational model that is more cost effective than physical prototyping. The design-build-test process can then follow with more confidence, while still in early stages of product development. Figure 12 shows these contrasting timelines – a physical prototyping vs. a simulation driven approach.
Reducing Risks for Testing Personnel
Physical testing carries inherent risks on testing personnel or their colleagues. Consider the range of medical device hazards, such as: sharp needles, heavy carts, hazardous materials (radioactive, biohazardous, chemical, or otherwise), pressure chambers, explosive batteries (e.g., lithium polymer), and high temperature systems. CM&S provides a controlled environment where the tester is not in harm’s way, allowing for a completely safe testing sandbox. Failure mode analysis and work with otherwise hazardous materials can be wide ranging and requires no precautionary preparation, allowing engineers to explore worst-case scenarios without the potential for catastrophic outcomes to health and safety. Within this environment, companies engaged in testing and verification can enhance safety and minimize potential liabilities for their employees. CM&S serves as a proactive approach to exploring risk assessments, leading to more robust and reliable designs and reducing the likelihood of costly failures in real-world applications, where these hazards can exist for the public more broadly.
Ensuring Quality in CM&S
Acknowledging all the value CM&S can provide to testing for medical device development, how do we know we can trust the results of these models? Thankfully, standards and regulatory agencies have been working on refining the now broadly accepted guideline to assess the reliability and quality of CM&S for medical device development – ASME V&V 40.
Safety, reliability, and effectiveness are paramount in the dynamic world of medical device development. CM&S offers a virtual testing ground for prototypes; however, to ensure these models provide the necessary accuracy, a structured framework is essential. The ASME V&V 40 standard, developed by the American Society of Mechanical Engineers (ASME), stands as a beacon for Verification and Validation (V&V) in computational modelling. Several aspects of the standard are described in this section to provide relevance for its use in increasing CM&S quality for medical device development.
Adherence to Industry Best Practices
ASME V&V 40 sets forth a comprehensive set of guidelines and best practices for verification and validation in computational modelling. It was written and continues to be reviewed and updated by industry professionals from leading companies and the FDA. By adopting this standard, engineers and medical device professionals align themselves with a widely recognized and respected framework, ensuring that their models undergo rigorous scrutiny.
Structured Validation Process
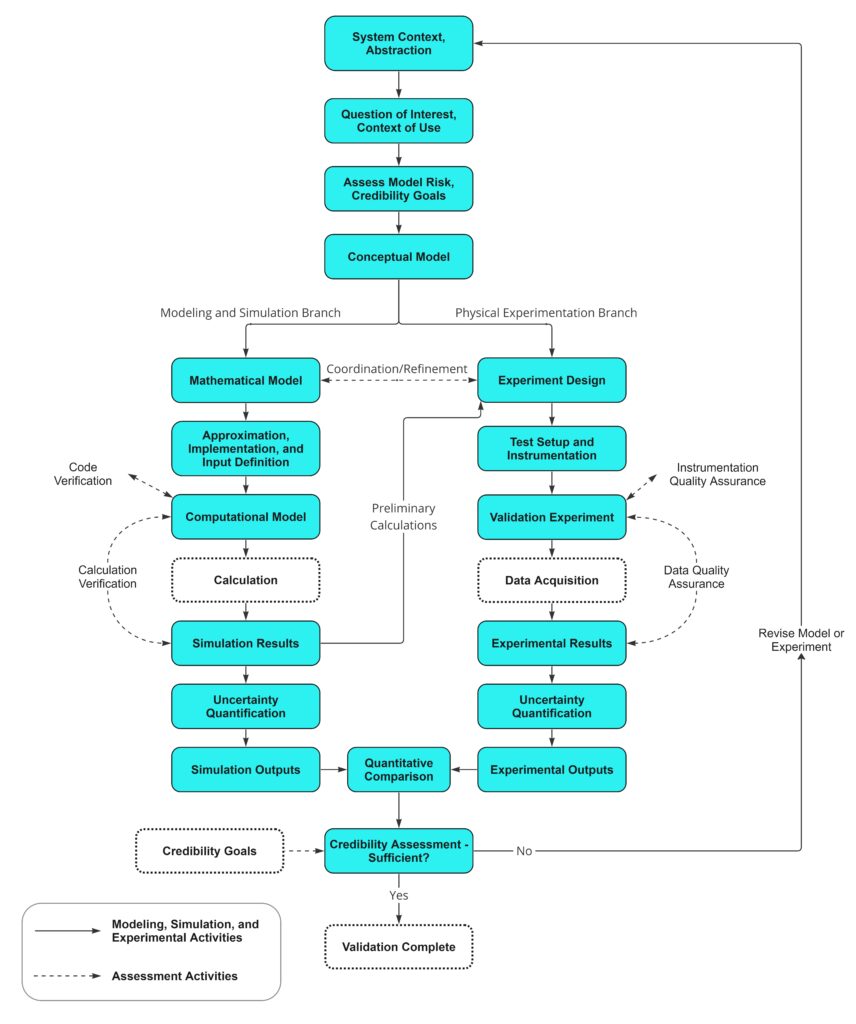
The standard lays out a systematic approach to model validation, encompassing steps such as model conceptualization, data generation, and uncertainty quantification. It measures these results against appropriate bench tests, allowing for real world comparison and refinements to be made to either the CM&S or bench tests. This approach empowers engineers to assess the robustness of their models, and review if real-world scenarios are represented. Structuring these assessment activities through V&V 40 framework mitigates the risk of oversight or haphazard validation efforts, resulting in models that are thoroughly tested and refined. The StarFish Medical CM&S V&V process flow chart is shown in Figure 13, and shows how this process takes place.
Uncertainty Quantification
If the output from CM&S has a high impact in a critical decision (e.g., risk to patient or users), its uncertainty should be quantified. ASME V&V 40 emphasizes the quantification of uncertainties inherent in computational models. This is paramount in medical device development, especially where predictions are critical for device efficacy and the safety of patients and users. By systematically addressing uncertainties, stakeholders are able to make informed decisions, being assured of model accuracy and validity to enable the safety of patients while furthering the advancement of medical device development.
Sensitivity Analysis
Computational models can be scaled with ease, allowing for parametric space to be mapped. Once available, sensitivity analysis can be employed to understand how different factors influence the device’s performance and compliance. This aids in making informed design decisions and provides insights into which parameters have the most significant impact on meeting device requirements. Consider cases where a physical component is stressed near to failure but shows no detectable effect. If the same test were to be brought into a CM, the simulations could provide comprehensive failure assessment. This could be alongside context from different parameters’ influence and provide justification for safety factors after assessing the worst cases (e.g., force and temperature limits).
Cost-Efficiency and Resource Optimization
Systematic verification and validation can provide early identification of model weaknesses or shortcomings. This also occurs within the bench testing branch, as indicated in the early-stage process cross checks in Figure 13, ensuring both systems are appropriately refined for the given study. Therefore, their outputs are better understood and easier to revisit if needed for refinements to studies or new inquiries. The overall approach to providing early-stage insight minimizes costly late-stage revisions or redesigns and optimizes project resources for high value development. By enhancing the confidence in computational models, ASME V&V 40 enables a more streamlined and cost-effective product development cycle.
Fostering Stakeholder Trust
Regulatory bodies in the medical industry expect robust evidence of a device’s safety and efficacy. They are included in a diverse array of stakeholders, including clinicians, patients, investors, and healthcare institutions. ASME V&V 40, which was developed with FDA input and oversight, provides a transparent and standardized methodology for demonstrating compliance. For critical outputs from CM&S, ASME V&V 40 can be used to prove rigorous quality standards were followed. This fosters stakeholder confidence and assures them of the developed device’s reliability and safety, allowing for smoother approvals through regulatory agencies and subsequent ease of market access.
Having established the importance of quality in CM&S and the standard for assessing its use in medical device development, it’s important to consider the role of regulatory bodies and their thoughts on CM&S in the industry.
The Future of CM&S in Medical Device Design
Rapid advancement in technology has allowed us to process huge amounts of data. With recent refinements in methods such as GPU calculation integration, parallelization, multicore machines, and high-performance computing, this capability is improving further [9]. As the technology has evolved, physics-based simulations are providing digital evidence for submissions by solving increasingly complex problems that were not previously feasible. In the wake of all these developments, it’s important to consider the future of CM&S and its application within medical device development and healthcare.
Virtual Patient
As discussed previously, simulation-based medical device development can provide multiple iterations of designs in short periods of time enabling potential designs to be refined and de-risked quickly. The effect of materials, geometric parameters, chemical composition, concentrations can be assessed at early stages of the design.
The next stage can be testing these novel designs on virtual patients. A virtual patient is a digital avatar that represents a patient model based on data obtained from millions of real patients. A virtual patient can also be a focused data set, such as specific regions of anatomy or biological systems like a gastrointestinal tract. Using this data allows researchers to generate patient models based on aspects such as ethnicity, size, gender, and age for a particular study and to test the efficacy of the device for the target population. As these methods evolve and become integrated into the development process, CM&S and virtual patients will completely disrupt the way medical device companies perceive clinical trials.
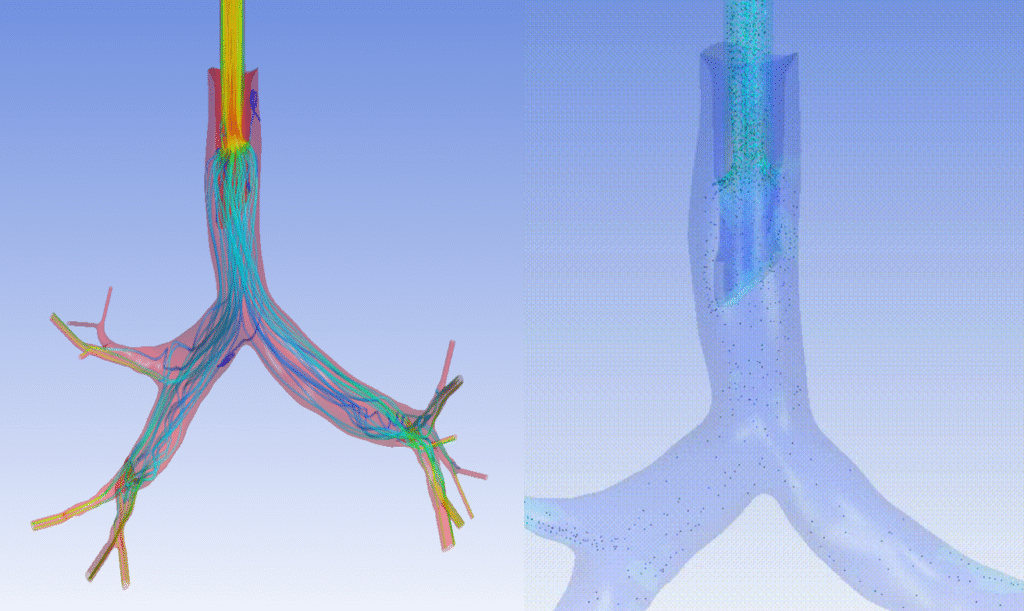
Imagine the impact from testing different ventilator designs on virtual Covid-19 patients in silico. Companies could reduce the length and risks during clinical trials on patients by incorporating biological variability to their models, providing efficacy of their devices across populations. Figure 14 provides an example of a lung’s upper region anatomy and the insight provided when parametric vectors are visualized. Patient, animal, and bench models would have difficulty approaching the resolution or parametric scalability (e.g., anatomical variation) of these in silico methods.
A medical device development ecosystem comprising virtual patients will speed up medical device development in a more comprehensive way by shortening the time to market, lowering development costs, and providing an impetus for development of innovative and disruptive technologies.
Digital Twin
In the distant future, the virtual patient concept will be extended further to a “digital twin”. While a virtual patient allows for testing of medical devices in typical models, a digital twin will tackle the concept of individual medical device development head on for personalized medicine.
A digital twin is a virtual replica of a human with the ability to “learn, adapt and improvise” based on the data received from the subject. To develop a reliable digital twin, quality data with resolution varying from cell to human body level needs to be collected with integrated technology (e.g., monitoring wristwatch). The ultimate digital development platform would incorporate personalized medical needs and be able to provide comprehensive information that is not yet possible. For instance, a digital twin could enable predictions of a device’s long-term effects, how it responds to changing environments, and its failure modes.
To summarize, In the wake of all the technological advancements, it’s important to acknowledge these new opportunities to take advantage of them in the near future.
Conclusions
CM&S is rapidly becoming a cornerstone in medical devices development. Its predictive capabilities, efficiency, and risk mitigation benefits are a compelling alternative to physical testing. Embracing CM&S not only leads to cost savings and faster development cycles but also fosters a culture of innovation and excellence in medical device design and manufacturing. Regulatory agencies are leading the increase in CM&S adoption, providing standards development to encourage quality and smoother CM&S-inclusive regulatory submissions. ASME V&V 40 is key to assessing CM&S quality and should be the standard used to guide any critical CM&S used in medical device development. The future promises an eclipse of traditional clinical trial methods by providing better platforms to assess safety and efficacy at scale. As the field continues to advance, integrating CM&S into the assessment of medical devices process will be instrumental to achieving higher levels of medical device safety, reliability, and compliance, all while decreasing time to market for the benefit of stakeholders and society.
References
[1] A. F. Frangi, “Unlocking the power of computational modelling and simulation across the product lifecycle in life sciences – A UK Landscape Report: Sounder, safer, faster and more sustainable innovation and regulatory evidence of medicines and healthcare products,” INSILICO UK, Sept 2023.
[2] Government of Canada, “Serious adverse drug reactions and medical device incidents reported by Canadian hospitals: Monthly summary,” 03 11 2023. [Online]. Available: https://health-infobase.canada.ca/hospital-adverse-events-dashboard/#a7. [Accessed 27 11 2023].
[3] FDA, “Healthy Innovation, Safer Families: FDA’s 2018 Strategic Policy Roadmap,” FDA, 11 01 2018. [Online]. Available: https://www.fda.gov/about-fda/reports/healthy-innovation-safer-families-fdas-2018-strategic-policy-roadmap. [Accessed 27 11 2023].
[4] T. Morrison, “How Simulation Can Transform Regulatory Pathways,” FDA, 2018.
[5] M. Andress, “Class II Recall Analysis,” in OMDRHO Virtual Conference, June 23, 2021.
[6] S. Moutinho, “Researchers and regulators plan for a future without lab animals,” Nature, no. 29, pp. 2151-2154, 2023.
[7] T. M. Morrison, “The Role of Computational Modeling and Simulation in the Total Product Life Cycle of Peripheral Vascular Devices,” J Med Device, vol. 2, no. 11, 23 01 2017.
[8] ASME, Assessing Credibility of Computational Modeling through Verification and Validation: Application to Medical Devices, ASME, 2018.
[9] T. M. Morrison, “Advancing Regulatory Science With Computational Modeling for Medical Devices at the FDA’s Office of Science and Engineering Laboratories,” Frontiers in Medicine, vol. 5, 25 09 2018.
Nathan Muller is a StarFish Medical Mechanical Engineer – Analysis and Design. His focus is in simulation engineering using computational modelling. As part of a design and development team, he frequently leads the development of mechanical design and device integration across disciplines, including targeted optimization and derisking activities through computational modelling and simulation (CM&S).
Muhammad Jamil, PhD, is a Computational Fluid Dynamics (CFD) Engineer in the Analysis and Design group at Starfish Medical. As part of the design and development team, he specializes in using Computational Modeling and Simulation in making design decisions and to accelerate the medical device development.
![Figure 1 – Med Device and Pharma: total market vs. in silico enabled [1]](https://starfishmedical.com/wp-content/uploads/CMS_Figure-1-3.png)