Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Today, 85% of the top 50 healthcare companies use Computational Modeling and Simulation (CM&S) to develop their products and processes. Whether it’s refining overall device parameters or optimizing critical requirements, engineering simulations help reduce development timelines and enhance design exploration.
-
Computational Modeling and Simulation (CM&S) for medical devices has become a pivotal tool across the medical device industry, complementing and often enhancing traditional bench testing and clinical studies.
-

In a sophisticated world of ever increasing complexity, we need our tools to evolve alongside us and assist in complex decision making, allowing us to understand the consequences of choices ahead. Computational Modelling and Simulation (CM&S) is emerging as an essential tool in building evidence for medical device development.
-
Aerosol Drug Delivery Systems, including Aerosol-based pulmonary-delivered drug devices, offer significant value by enabling targeted drug delivery directly to the respiratory system.
-
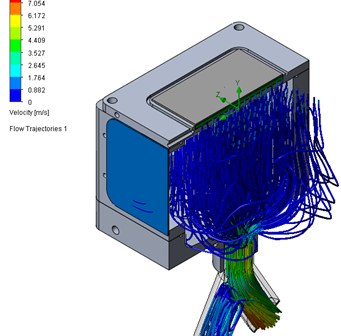
The impact of vibration on medical devices can be understood through a combination of vibration analysis and testing. This article reviews medical devices most susceptible to vibration, provides basic theory and methods of vibration analysis, and summarizes the benefits of using modal analysis when developing medical devices.
-
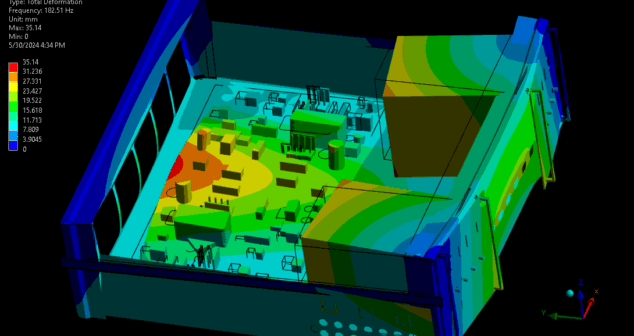
Importance of using Computational modeling thermoplastic properties to predict and validate thermoplastic properties in a medical device.
-
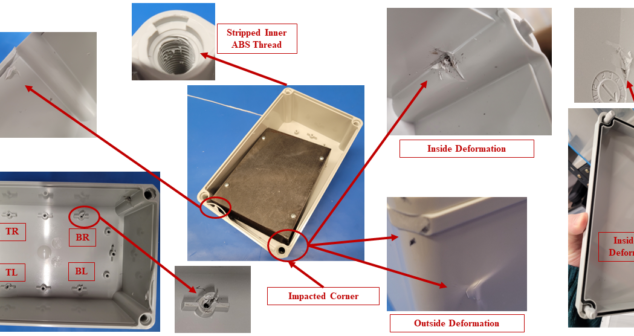
In silico medical device drop testing offers scalability, fast-tracking of iterations, and the ability to review hard-to-detect failure modes.
-
3D Printed Parts Streamline Development provides context for designers using 3D printed parts as a part of their market-ready medical devices.
-

FDA Guidance on Additive Manufacturing (AM) – offers new ways to integrate parts not only for prototypes, but for final products.