
Medical Device Sterilization Methods and EtO
Medical Device Sterilization and Categories
Sterilization is a critical process in the medical device industry as it provides a reliable way to ensure that devices are free from harmful microorganisms when they are used on patients. This blog talks about the categories of sterilization currently used on medical devices in manufacturing settings. It also addresses concerns surrounding the use of ethylene oxide (EtO), an indispensable method for sterilizing heat and moisture sensitive devices.
As per FDA, there are mainly two categories of sterilization methods currently used in manufacturing settings: established and novel.
Established Sterilization Methods
- Established Category A:
- This category consists of methods that have a long history of safe and effective use in the medical device market demonstrated through literature, 510(k) clearances or premarket approvals (PMAs), and satisfactory quality system inspections.
- There are voluntary consensus standards recognized by FDA that address the development, validation, and routine control of these established methods.
- Some examples of methods of sterilization that are considered Established Category A are:
- Ethylene Oxide with devices in a fixed, rigid chamber
- Moist Heat or Steam
- Dry Heat
- Radiation (e.g., electron beam, gamma)
- Vaporised Hydrogen Peroxide
- Established Category B:
- This category consists of methods that do not have FDA-recognized dedicated consensus standards, but do have published information on development, validation and routine control of medical devices.
- Some examples of methods that are considered Established Category B sterilization are:
- Flexible bag systems (e.g., ethylene oxide in a flexible bag system, diffusion, injection)
- Ozone
Novel Sterilization Methods
- A sterilization method is considered to be novel when the specific process does not appear to have been evaluated by FDA, either because the parameters of an FDA cleared sterilizer have been altered or because process validation data has not been evaluated and found to be adequate in previous cleared or approved submissions.
- These methods are considered newly developed for which there exists little or no published information.
- In such methods, there are no history of comprehensive FDA evaluation of sterilization development and validation data through an FDA -cleared 510(k) or approved PMA for devices, and no FDA recognised dedicated consensus standards on development, validation and routine control.
- Involvement of Chemical Component in Sterilization:
- Any sterilization method that uses chemical(s) that have not been previously cleared or approved by FDA as a chemical sterilant or have not been identified in the scientific literature as a chemical sterilant, would be considered novel.
- Moreover, if a sterilization method uses a chemical combination that has not been previously cleared or approved by FDA as a sterilant, it would be considered novel (even if the individual chemicals in the combination have been previously cleared or approved separately as chemical sterilants).
- Some example methods of sterilization that are considered novel:
- Ultraviolet light
- Sound waves
- High intensity light or pulse light
- Vaporised peracetic acid
- Microwave radiation
Recent Concerns Surrounding Ethylene Oxide Sterilization (EtO)
Overview of Ethylene Oxide Sterilization (EtO) and Usage
- Ethylene oxide sterilization is widely used in the medical device industry to keep devices safe. For many devices, this could be the only sterilization method that sterilizes effectively without causing any damage to the device during the sterilization process. It is mostly used for temperature sensitive devices (e.g., catheters). A range of devices, including products for general health care practice and products used to treat specific areas of the body, can be sterilized using ethylene oxide.
- Ethylene oxide sterilization is mainly applicable for devices that allow the gas to penetrate them. Hence, it is also important to consider documentation like AAMI TIR17:2024; Compatibility of materials subject to sterilization to ensure that the material/product is compatible with the selected sterilization method (i.e., sterilization method should be considered during design input).
- Ethylene oxide is used in health care facilities to reprocess critical and semi-critical items that are moisture or heat sensitive and cannot be treated by steam sterilization.
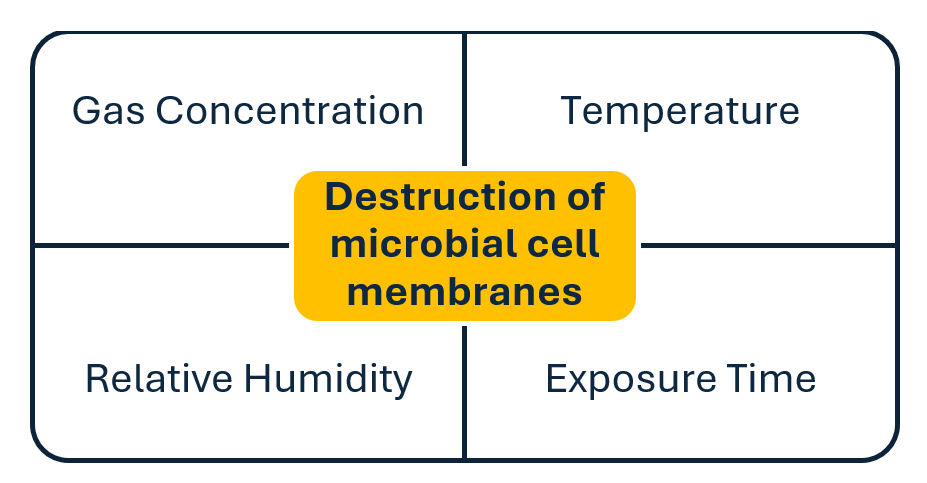
- The four parameters involved in ethylene oxide sterilization are: gas concentration, relative humidity, temperature, and exposure time. Sterility is achieved when an ethylene oxide gas molecule reacts with and causes destruction of the microbial cell membranes.
Concerns Surrounding Ethylene Oxide Sterilization
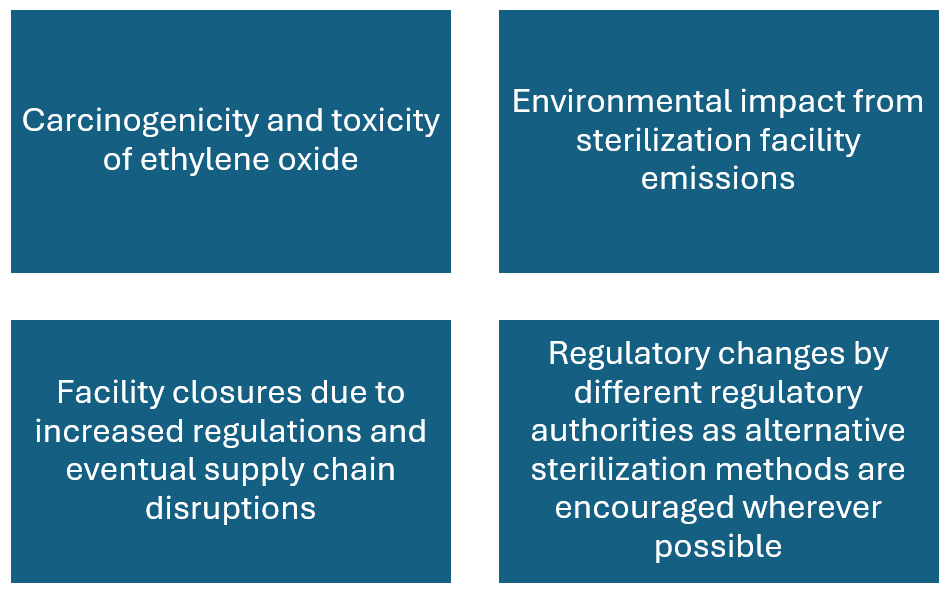
Ethylene oxide has come under scrutiny due to environmental and health concerns, despite its efficacy.
The major concerns can be summarised as follows:
Standards and Regulatory Bodies:
Voluntary Consensus Standards
- Long term and occupational exposure to ethylene oxide has been linked to cancer (ethylene oxide is carcinogenic to humans when inhaled).
- Two voluntary consensus standards (ANSI AAMI ISO 11135:2014 and ANSI AAMI ISO 10993-7:2008) that describe how to develop, validate and control ethylene oxide sterilization processes for medical devices and the acceptable levels of residual ethylene oxide and ethylene chlorohydrin left on a device after it has undergone ethylene oxide sterilization.
- These standards help ensure levels of ethylene oxide are within safe limits.
Environmental Protection Agency’s Role
- The United States Environmental Protection Agency (EPA) reviews and enforces the Clean Air Act regulations for sterilization facilities that emit ethylene oxide to ensure that the general population is protected from major risks caused by the emissions of ethylene oxide.
FDA’s Role
- FDA efforts regarding concerns surrounding ethylene oxide mainly address two issues:
- Bring up new and innovative methods to sterilize medical devices.
- Reduce the potential impact of ethylene oxide on the environment and public health.
- Additionally, FDA has developed programs encouraging new sterilization methods and allowing manufacturers to make changes to or advance alternative ways of sterilizing approved devices (including changing radiation sources) in a least burdensome regulatory approach.
Conclusion
Medical device sterilization is an integral part of the medical device industry and patient safety. It is essential that its effectiveness is balanced with environmental and health concerns. Traditional methods like steam and radiations serve many needs, but ethylene oxide remains indispensable for sterilizing heat and moisture sensitive devices.
Industry must navigate growing concerns about EtO use by embracing safer practices and adopting alternative pathways of sterilization. By staying informed about advancements, challenges and latest regulatory landscapes, manufacturers and health care providers can ensure the continuous availability of safe methods to sterilize medical devices for years to come.
As a member of the QA/RA team, Bhavyashree is dedicated to ensuring product quality and compliance. With a keen eye for detail, she strives for excellence within the field of quality assurance and regulatory affairs.
Images: Adobe Stock
Related Resources


Nick and Nigel walk through how sterile disposables are processed and verified before they reach the field.

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.

The sandwich ELISA assay is one of the most common ELISA formats used in diagnostics. Nick and Nigel walk through the method step by step using simple visuals and plain language.