
Six tips for developing a “lab-on-a-chip” (LOC) solution
Microfluidic systems, also referred to as “lab-on-a-chip” (LOC), “biochips”, or micro-total-analysis-systems” are widely used in the Biotech industry. This blog shares six tips for developing a “lab-on-a-chip” (LOC) solution.
Miniaturized systems can carry out entire protocols traditionally performed in a laboratory. Sample pretreatment, sample and reagent transport, mixing, incubation, separation, detection and product collection can all be performed automatically on a single LOC system. Applications range from Point-of-care (PoC) diagnostics, to single-cell analysis (e.g. Biocept) and even disease modelling in tissue engineering (a living system on a chip).
- Determine your intended final product early on. Understanding your product’s market and the users it will serve is critical. An important part is the panel of tests and what your product will deliver. What are the possible features and benefits? Is it faster, cheaper, or does it have a lower detection limit? You need to define early on what the targeted product cost to purchase will be. This will drive much of the trade-off discussion. It comes down to a “scope triangle” of three conflicting priorities: Cost, Time and Quality. You will have to pick two as you cannot have them all.
- Take a system approach. There are multiple aspects to the solution (chemistry or assay, cartridge and reader/instrument), but you cannot separate them completely as there is ultimately an interplay between them. The initial challenge is to decide where functionality will reside – in the instrument/reader or the cartridge. If it is in the cartridge, then the challenge becomes how to make it manufacturable at a price aligned with the market. The alternative is to balance functionality such as pumps and fluid control in the instrument/reader to simplify the cartridge. Ultimately, you need to keep testing at the system level as the various elements are being developed and these trade-offs are explored.
- Test with real samples early. If the final solution is going to perform a test on blood, then test blood as early as you can. A complex matrix such as blood will be the source of many issues. These issues need to be identified and resolved as early as possible. Ideally your development partner should have access to Biological Safety Levels (BSL) Level 2, or BSL2, laboratories and have an experienced scientific team to support this testing. Once you have demonstrated standards and controls perform acceptably in the new format, then use blood. You can simulate certain aspects of the design and prototype, or 3D print elements, to explore usability. However, the complex interplay between the various materials (sample, reagents, cartridge walls), and factors such as the surface tension, air, bubble generation and their impact on chemistry performance are amplified at the micro-fluidics scale. Understanding what these interactions are, and how they manifest, can take a lot of time and effort. It is very important to have some mechanism to observe fluid behavior, not just the collation of the chemistry signal or results, as this will provide insight into possible solutions.
- Align with the commercial scale cartridge manufacturing process as early as you can. Changes to the microfluidic format will affect chemistry performance. Endeavor to stabilize the format early. This will require the development of a pilot scale cartridge manufacturing and reagent loading solution. Generate a relatively small number of cartridges for early testing. These cartridges will likely be manually filled with reagents for the early proof-of-concept testing.
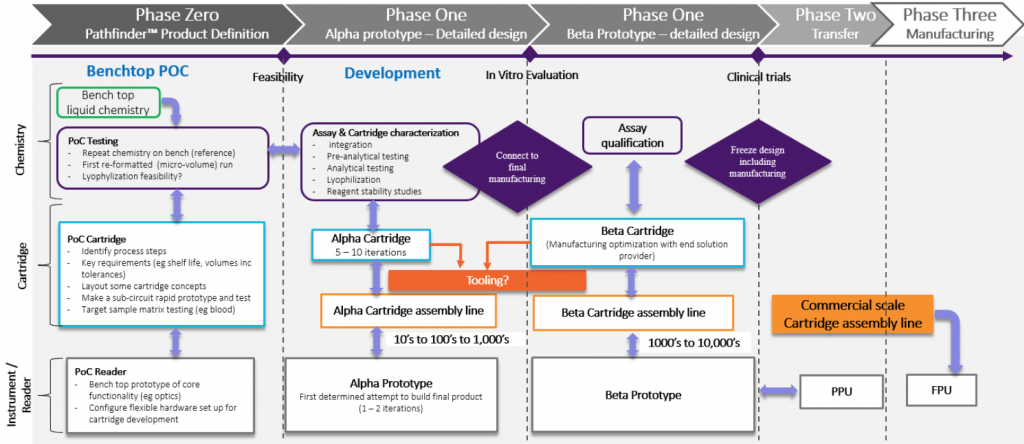
- Start to explore reagent stability on the cartridge as soon as you can. As an example, lyophilization has been around a long time, but don’t think that makes it easy to implement for on-board cartridge reagent storage. As the reagent volumes decrease, dried reagents are required to enhance cartridge shelflife. This creates the challenge of dispensing the reagent into the cartridge, drying the reagent and then sealing. Don’t forget that most plastics “breathe”. Is your cartridge really sealed? Material selection is critical, and generally limited, so if you want to explore novel materials, it will take time.
When you move to injection molded components, the method of how they are produced will impact chemistry performance. Chemicals such as mold-release agents may have animal derived components or other elements that can interfere with the assay chemistry. If the chemistry is a molecular assay, then any plastic component will need to be DNA, RNA, DNase and RNase free. Use a good vendor familiar with these challenges. The initial small runs or parts may require post-production cleaning and manual assembly in a cleanroom.
- Plan your supply chain of system components carefully to support clinical testing. A systems approach to components such as reagents (do they need to be GMP grade?), cartridges loaded with reagents, readers and instruments, is required as each component must remain in sync with the overall system development and all need to be aligned with the regulatory approach. You do not want to over invest in a particular aspect, such as the cartridge design ahead of the reagent or reader development. For a diagnostic system, you will require each element (reagents, cartridge and reader) to be appropriately designed for the stage of development. For a reader/instrument required for use in clinical trials in the USA, you will need the design to have been developed under FDA 21 CFR Part 820.30. Finding a partner that can support you in these different aspects can be a challenge, but a partner with in-house scientific and regulatory teams is big advantage.
Developing a “lab-on-a-chip” (LOC) solution brings unique challenges and benefits. I hope the tips and insights in this blog help. I’d enjoy hearing other tips and insights or discussing LOC projects with readers.
Brian Hanrahan is a former Biotech Program Director at StarFish Medical. Brian has more than 15 years of experience delivering successful product development in the biomedical and cell therapy industries.
Lead Image: StarFish Medical