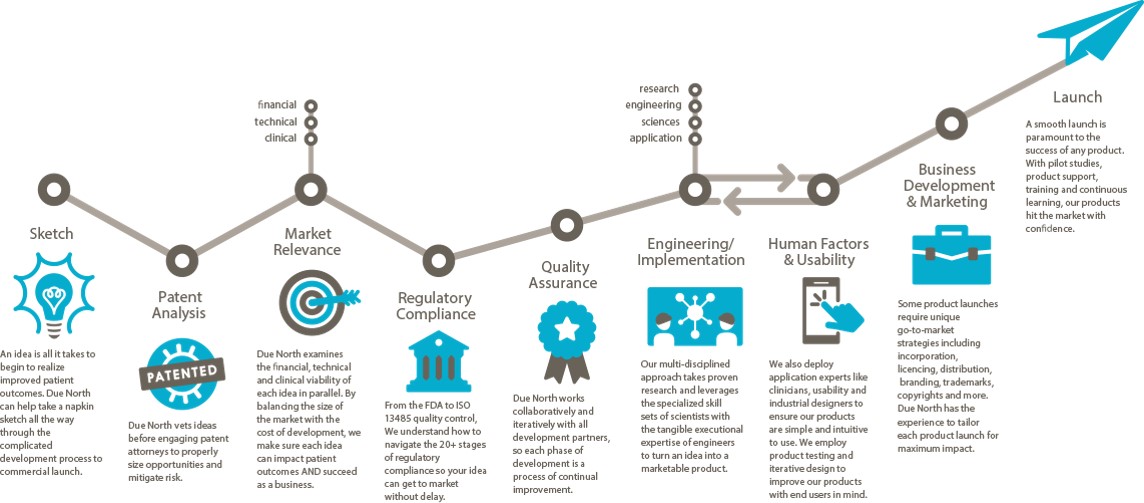
Sketch to Launch commercialization process for medical devices
I described the Sketch to Launch commercialization process that Brenda Edin and I use at Due North Innovation to provide commercialization services to the medical industry at the recent Medical Device Commercialization Playbook event in Vancouver BC. The following is a transcript of my talk at the event.
Due North is a rebranding and advancement of Baker Group, which has been business since 1999 with a Sketch to Launch commercialization process. We take a lot of technology from our own inventions, universities, national labs, hospitals, and entrepreneurs, then translate the technology into something that is commercially viable.
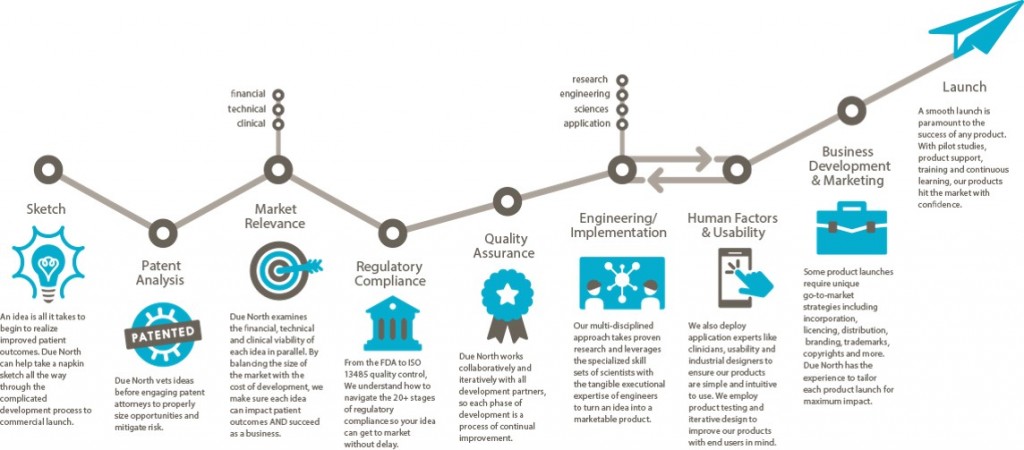
Sketch: One project even came to us on a napkin. It was from Pacific Northwest National Laboratory to create a new way of breast imaging using through-transmission ultrasound. We created the holography methodology so that images could be displayed for physicians to look at. We did that in 9 months. We got it through the FDA in 47 days. We are 60 some projects since 1999. All of them went through the FDA and all went through the process. We were charmed. [Editor’s note: Watch a clip from the Q&A session where Michael discusses the overlap and synergies between some of those 60 technologies and products.]
Patent Analysis: Make sure the Intellectual Property (IP) is under control. IP is the way we make money in our group.
When we are introduced to a technology or invent it on our own. We first do the IP. We then look at the marketplace.
Market Relevance: Is this thing going to be relevant in the market? Is it going to change how the delivery of medicine is being done? Is it going to improve how things are going to be done? Is it going to improve the quality of life? We have to prove to ourselves that these things are occurring or we won’t go forward. Unfortunately we see a lot of amazing solutions, but no one can afford to pay for it, or it costs hundreds of millions to get to market and people will only pay $10 for it.
We do a financial viability model. It’s different than a budget or a pro forma. This is something we do to evaluate the efficacy relative to what the market will bear and determine if we can build something for a reasonable price.
So we match up the price with the technical team (we use StarFish for implementation).
Regulatory Compliance: Find a Regulatory champion. We expanded from local experts to Hogan Lovells in Washington DC and San Francisco exclusively. We also use StarFish to help us with this. They helped us move LEVO through the FDA in 91 days. We shifted from local quality and regulatory experts because the timeline to educate the FDA on your project needs to be shortened. Every second you spend educating or meeting compliance with the FDA is time away from the market. We use regulatory attorneys to step it up. Sounds expensive, but it’s not. You just have to find the right attorneys that interface with the FDA on an ongoing basis. A relationship with the FDA is incredibly important. It’s something we hold dear. Answer their questions, don’t argue with them. We apply for pre-submission meetings with the FDA. It has been very valuable. It’s a process and a bit of a risk for negative exposure, but we haven’t had a problem.
Quality Assurance: You have to have a quality system in place. We use StarFish. A QMS is imperative to a successful venture and a successful launch of your product. It saves time. It saves money. And you get a much, much better outcome if you embrace the quality system. The last thing you want to do it to wake up 10 days before submitting to the FDA and go, “I need a procedure for something.” We do all of this stuff in the front end.
Engineering/Implementation is incredibly hard. The iterative part requires a lot of project management, a lot of attention to detail and having a lot of creativity. I can’t stress enough how important this in your engineering team (and why we use a lot of physicists for this.) Creativity and solving problems is all we do all day to make sure something will work appropriately. Going through that process is a huge effort and one where you have to have the right people on the bus. Creative teamwork is very important within your engineering team.
Human Factors & Usability: If you have a product that touches a patient or has someone operating it, the FDA has come up with a new standard that they police closely. Human Factors use case scenarios must be documented, in place, and demonstrated effective within your device whatever it is—software included. Knowing how to do that and having the right people to do it is very important. Brenda Edin and her team are the human factors resource (among many other things) for Due North. We’re one of the few firms that do it in-house. We also outsource some work to Battelle in Ohio.
The other commercialization process component we do on all products is simulations. It has helped us with our FDA submissions. We send them the videos, protocols, reports, and outcomes throughout the simulation process. It just clears the desks in terms of usage, human factors, and the safety of the device. Cedars-Sinai does this really well as does OHSU in Portland Oregon.
Business Development and Marketing: Now you’ve made it. Now it’s got to go someplace. That’s a whole science in and of itself. What we do in our commercialization process that’s a little bit different is that all the engineers, regulatory people, QMS people, everybody that’s in the front, the patent attorneys, and corporate attorneys–everybody participates in developing the business and launch plan because it touches every one of them. We fast track that as well. We went from zero to a launched product at the beginning of this year in a little under two years using that methodology. If we can’t turn something in 24-36 months, we pass on it.
We sell and buy technologies so we sit on both sides of the Business Plan. No one reads them. Except to check on a figure. They are a box to tick off. But all of the things that are in the plan that are historic, you need to know how to do intimately. We call it more of a company fact sheet. It builds around the financial viability showing investors clearly how they are going to make money. It’s used as an instrument to argue with the investment community on how to evaluate your company. It comes down to how much someone is going to pay you for your technology.
The only way you can defend your valuation is if you do all the homework and can demonstrate it. That’s been the key for our success and commercialization process. We’re not a fund so we raise a lot of money for projects.
Launch: At the point of the product launch we get out and start on a new project.
Michael Baker is a Partner at Due North Innovation, CEO of Otoharmonics Corporation, Co-Founder of Applied Exergy, Inc., and Founder of Home Dialysis Plus, Ltd., Portland OR.
