Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

How to handle recent medical product development regulatory changes under the 21st Century Cures Act and device exemption list.
-

Start early to avoid surprises when preparing for MDR 2017/745. The new EU medical device regulations will come into full force in Q2 2020.
-

Tips to manage competence and training, keep up to date and ensure personnel are trained and competent to perform their duties.
-

MDSAP Medical Device Single Audit Program audits will help ensure compliance with QMS and reduce the audit/inspection burden.
-

11 Lessons learned from medical device project implementations cover a range of topics from regulatory and quality to development.
-

Overview of FDA Regulation of E-cigarettes Deeming Tobacco Products to be subject to the Federal Food, Drug and Cosmetic Act, as Amended by the Family Smoking Prevention and Tobacco Control Act.
-

Digital Health communication technology cost and deployability for medical devices features a discussion of StarFish engineers
-

Implications of Device Generated Data using medical devices capable of data generation and uploading into digital health.
-
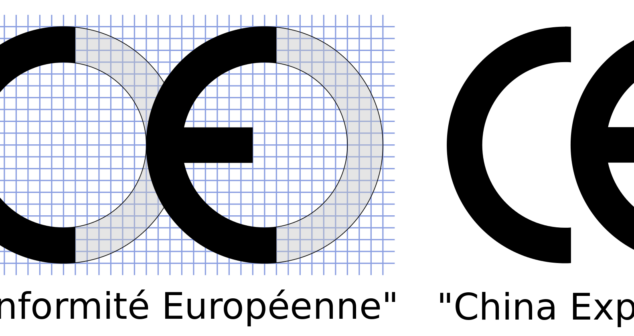
CE vs China Export Mark Explained – Discover meanings, regulations and visual layout of the two marks to avoid costly mistakes.