Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
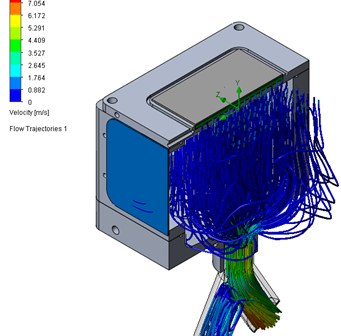
Aerosol Drug Delivery Systems, including Aerosol-based pulmonary-delivered drug devices, offer significant value by enabling targeted drug delivery directly to the respiratory system.
-

In this episode of Bio Break, Joris and Nick explore the increasingly important world of drug-device combination products, discussing what they are, why they matter, and the unique challenges associated with their development. As modern pharmaceuticals become more complex, the role of engineered medical devices in delivering these therapies safely and effectively has never been more critical.
-
In this engaging episode of Bio Break, Nick and Joris dive into the complex world of platform technologies in product development, exploring the pros and cons of this widely discussed concept. From in-vitro diagnostics to point-of-care instruments, the term "platform" often sparks excitement—and sometimes hesitation. But what does it truly mean to build a platform-based product, and when does it make sense?
-

Nick and Joris dive into the surprising connection between rock bands and medical device safety. What do jelly beans and 1980s rock legends Van Halen have to do with developing safe and effective medical technologies? More than you might think.
-

Nick and Joris tackle a topic that’s more relevant than ever—raising funds for medical device development in today’s challenging financial landscape. With economic headwinds and cautious investors, startups and even established organizations face significant hurdles in securing funding. But there are ways to mitigate these challenges, and Joris shares strategic insights to align product development milestones with fundraising cycles.
-
Filter Integrity Biopharmaceutical Manufacturing explores the relationship between microbial retention tests and integrity tests essential for validating the effectiveness of filters in critical applications.
-

Review of FDA Guidance on Using ISO 10993-1 integrating biocompatibility evaluation within the risk management process, as outlined in the FDA Guidance Document Use of International Standard ISO 10993-1, Biological Evaluation of Medical Devices Part 1: Evaluation and testing within a risk management process.
-

Integrating service design with user-centered design when developing medical devices can enhance device usability, efficiency, and outcomes.
-

7 Tips for conducting Voice of Customer medical device research in Low- and Middle- Income Countries (LMICs) is an integral component in user-centric design and development. It is increasingly an expectation of regulatory bodies.