Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

How to get the most from your FDM 3D printer covers all of the key things to keep in mind when designing parts for FDM 3D printing.
-
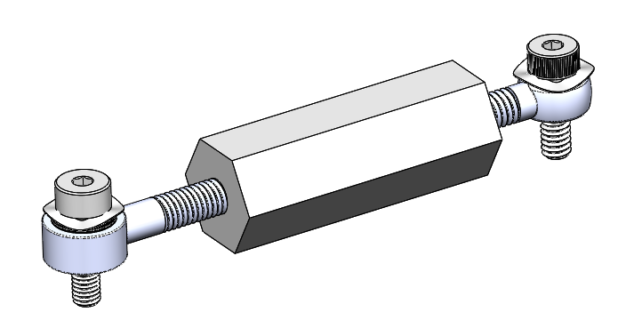
Design and construct a custom differential screw that is relatively inexpensive and easy to integrate with your system.
-
Discover how ERP Benefits Medical Device Commercialization from the perspective of a Mechanical Engineer and project coordinator,
-

Four questions one should address when considering Infectious Disease Diagnostics market potential by using public information.
-

5 simple tricks to reduce low volume supplier lead times and mitigate most medical device vendor supply time challenges.
-

Tips for tackling supply chain quality challenges in low volume medical device manufacturing from Kathy Young, Manufacturing Director, StarFish Medical.
-

Eight robust First-to-Market commercialization tips and insights addressing trade-offs when bringing a medical device to market.
-

Tips for a productive and successful EMC lab visit and assessment from the StarFish Medical Electrical Engineering team.
-

How to choose a medical device stepper motors, plus different types of stepper motor configurations and how to drive medical device stepper motors properly.