Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

9 tips for 510(k) submission along with lessons learned from a recent successful experience submitting a medical device to the FDA.
-

16 things to consider for the Medical Device Regulations (MDR Deadline) and changes you need to make in your Quality Management System.
-
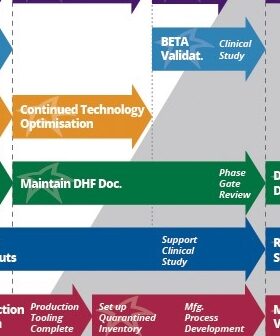
Why build a beta medical device? After a medical device receives regulatory clearance, it’s fairly burdensome to make changes. Beta device benefits are explained by experts.
-

Insights and observations that spark medtech innovation are brainstormed by team of medical device professionals at StarFish Medical.
-

Medical Device Playbook was conceived to inspire entrepreneurship and connect the many players. Here are commercialization Insights from attending the event.
-

Closing the medtech skills gap: Who better to teach medtech skills within academia than those who are earning a living with those very skills?
-

Medical device development experts share their biggest medical commercialization lessons learned over 20 years.
-

Michael May, President and Chief Executive Officer of CCRM discusses the intersection of medical devices in Regenerative Medicine.
-

7 interesting outcomes of the 2019 Health Canada Action Plan on Medical Devices initiatives and alignment with international regulators.