Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Five Steps to Medical Device Development outlines a process to follow that helps ensure successful commercialization.
-
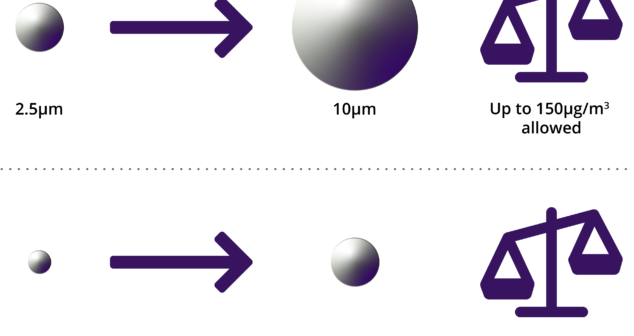
Pre-Testing for ISO 18562-2:2017; how to approach in-house pre-testing and add confidence to 18562:2-2017 formal testing.
-

Translating user and patient insight into tangible design offers tips and techniques for industrial designers and human factors researchers.
-
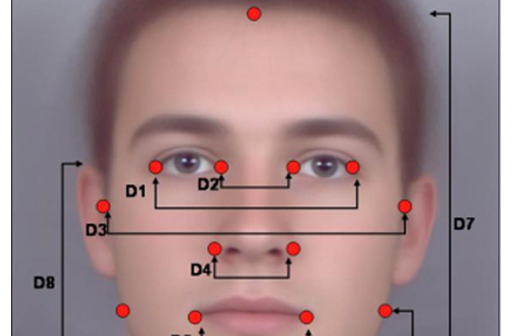
PM, EE, ME and ID/HF Manager insights and examples of how Medical Device Human Factors and ID impact Commercialization success.
-
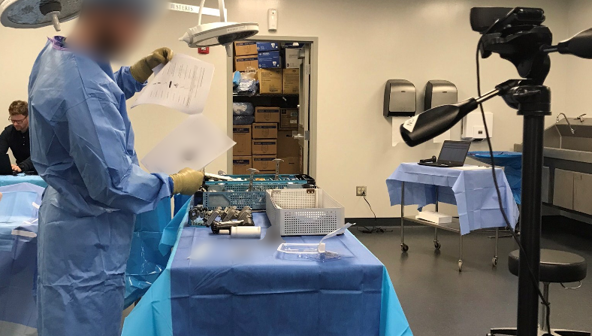
Effect of Formative Test Fidelity shows the importance of formative testing and its impact on medical device design team usability data.
-

Algorithm Aided Design (AAD) allows for new possibilities and opportunities in the medical market in terms of custom design and 3D printing.
-

Listening Strategy for Startups offers a three-pronged approach to stay competitive within the startup ecosystem.
-

Explanation of changes in medical device symbols and practical implications as a result of the ISO 15223-1:2021 Symbols Update.
-
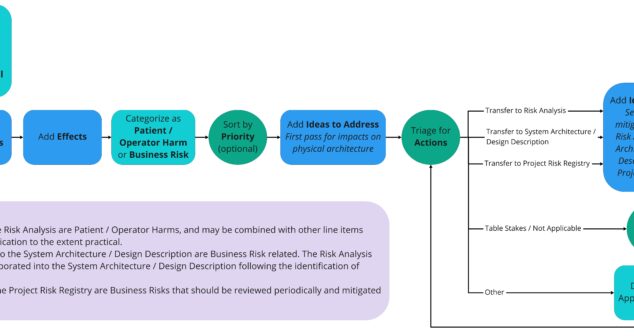
Using xFMEA as a Brainstorming Tool uncovers a powerful early-stage medical device design input tool used successfully at StarFish Medical.