
Medical Device Design for the Developing World
“We need to think about medical device as a system that delivers an operational capability.”
I saw a picture of a dumping ground for medical devices in Malawi. This made me wonder – why are there so many nonfunctional and broken medical devices in low-income regions of the globe?

It turns out that a significant percentage of imported medical devices in the developing world is out of service. The estimates for these broken devices range from 40% to over 70%. Compare this with only 1% in high-income countries [I. H. Marks et al]. While some of these estimates might not be accurate, one thing is true: an effective medical device built in North America can simply fail or go out of service in a place like Sub-Saharan Africa. Why?
Why do medical devices fail?
It is worthwhile noting that, most of the medical devices in low-income regions of the world are donations. The WHO guidelines for healthcare equipment donations states:
“In the Sub-Saharan Africa region, for example, a large proportion (up to 70 per cent) of equipment lies idle due to mismanagement of the technology acquisition process, lack of user-training and lack of effective technical support.”
What can we do about it?
On the Donation Side – Several small and large organizations have tackled this problem by better managing the donation process or providing technical training and services. Take, for instance, the Engineering World Health (EWH). This organization provides installation, training and repair for life-saving equipment in low-income countries. There are several other organizations working to improve the donation process. But, donation management and post evaluation of device operational success are one side of the story. We are also challenged by the device “design”.
On the Design Side – Robert Malkin clearly identifies the main design barriers: lack of spare parts, lack of required consumables (such as, ECG electrodes and test strips), lack of reliable power and water, public infrastructure and technical expertise. With the realization of the market values in low-income countries, there are now companies that manufacture devices considering the existing needs and operational barriers in those low-income regions. Such effective designs embody the frugal innovation process to meet the economic, environmental and delivery requirements of the particular region that they are going into.
A Systems Engineering Perspective
From a systems engineering perspective, a medical device is not just a device. Rather, it is a “capability system”. That is, a system which combines not only hardware and software, but all the other essential elements it requires to meet the needs of its targeted population and use environment. Among those are personnel, training, infrastructure, maintenance, environment, culture, leadership and policies. That being said, our success in building a medical device design for the developing world is about delivering a successful operational capability.
Let’s look into two successful designs that actually work for their targeted populations and use environments.
Uniject – Navigating Barriers in Vaccination
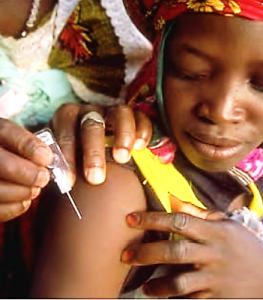
The first example is Uniject, a compact, pre-filled, auto-disable injection device developed by PATH for people with limited access to vaccination and medicine. Uniject requires a short training time, it is safe for self-injection and it generates less waste compared to multi-dose vials. With all these characteristics, Uniject navigates three main barriers on the way of vaccination in poor infrastructures: injection safety, vaccine wastage and access to immunization services. PATH describes what they do on their web site: “We distill cutting-edge medical technologies to their essence, then re-invent them as affordable, reliable, easy-to-use tools that work in places where resources are limited, power is scarce, or trained health care workers are few and far between.”
Fionet – Helping Health Workers Deliver an Accurate Diagnosis

Another frugal design example is Fionet. Rapid testing for disease such as malaria or Ebola in low-income regions of the globe is not an easy task. For one thing, there is limited access to centralized laboratory services. Hence, these diagnostic tests heavily rely on community health workers with limited supervision and controls. This in turn results in inaccurate test results.
Fionet system was designed as a mobile, cloud-based, low-cost diagnostic tool to address these challenges. Fionet does two things. First, it empowers community workers to deliver an accurate diagnosis regardless of their skill level. Second, it provides remote oversight of the diagnostic process. Currently, Fionet is being deployed for COVID testing in regions with limited access to centralized laboratory resources.
Final Note on Medical Device Design for the Developing World
A medical device is more than a device. Indeed, it is a system that delivers an operational capability to a targeted population and use environment. What is your strategy to design a successful “Medical Device-as-a-Capability”?
Arash Samimi is a Systems Engineer at StarFish Medical. He helps clients realize their creative ideas that can deliver values to healthcare in transformative ways. Prior to StarFish, he worked with early-stage medical device companies, built new industry-university partnerships, co-founded a social-mission startup and led fundamental research projects for defense and manufacturing industries. Arash holds a PhD in applied physics from Queen’s university.
Images: StarFish Medical