Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Proper research and communication with authorities in each country of interest will make Medical Device International Regulations smoother to navigate.
-

ISO/IEC 17025:2017 includes many changes. Three points to keep in mind: more options, involvement of risk, updates in current technology.
-
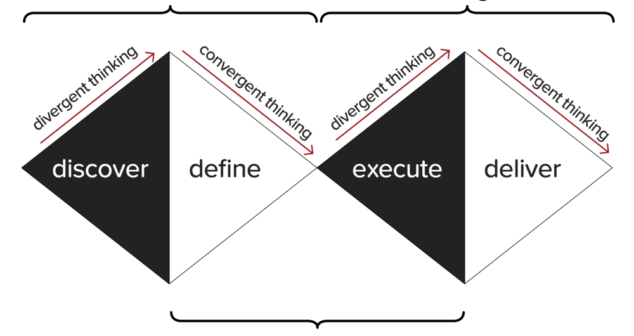
Kepner-Tregoe’s original Decision Matrix overview with tweaks to make it broader and more robust.
-

FDA Guidance on Additive Manufacturing (AM) – offers new ways to integrate parts not only for prototypes, but for final products.
-

11 medical device development mistakes that surfaced frequently in a variety of products. Avoid them to reduce cost and time to market.
-
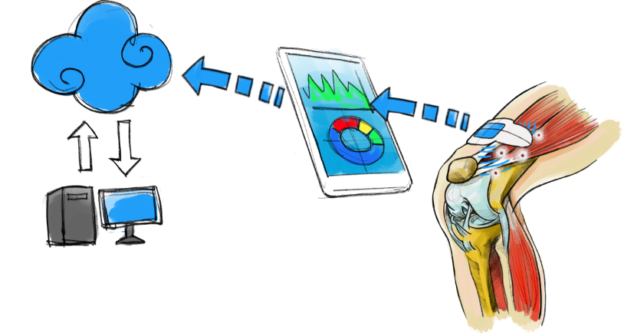
GDPR is designed to enhance the privacy and security of data subjects. Medical devices and GDPR cross paths in many areas.
-

Our employees' medtech resolutions include great ideas. What are your improvement plans? We'd love to hear and share them.
-

Top 10 StarFish Medical blogs of 2017 feature a mix of regulatory, engineering, and practical information and advice for all levels of expertise.
-

Enjoy our 2017 Readers' Choice Blogs and StarFish Medical articles, images, events and videos from our monthly newsletter.