Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Managing evolving requirements is a core competency for medical device technical specifications and understanding health care end users.
-

Open Source Software Medical Devices: Using OSS libraries within a Software System of a Medical Device or In Vitro Diagnostic Device.
-

Why design control is required for medical devices and is superfluous in the consumer goods design with advantages and disadvantages.
-

11 medical device development mistakes that surfaced frequently in a variety of products. Avoid them to reduce cost and time to market.
-

When selecting a method for developing an assay, whether it is for detecting bacteria or simulating fluid, the approach all depends on…
-
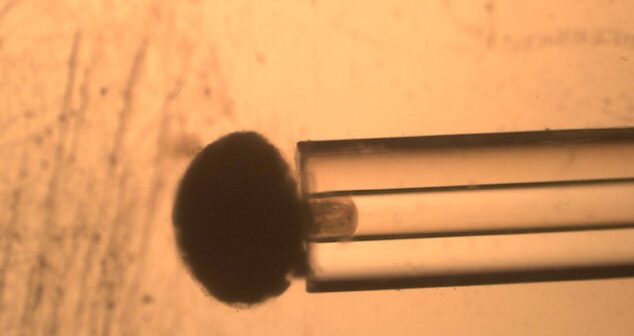
The technique of Micropipette Aspiration is an inexpensive way to measure the viscoelastic properties of cells.
-
Design control shouldn’t inhibit creativity; it should enable it by providing a safety net in the form of a system to fall back on.
-

Top 10 StarFish Medical blogs of 2017 feature a mix of regulatory, engineering, and practical information and advice for all levels of expertise.
-

Enjoy our 2017 Readers' Choice Blogs and StarFish Medical articles, images, events and videos from our monthly newsletter.