Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
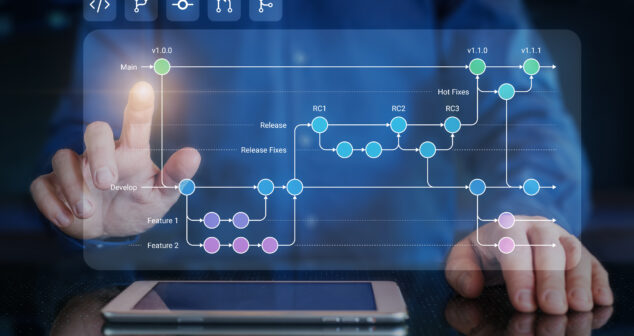
Medical device software is very diverse. In all this variety, one technology is ubiquitous: Git! Three eye-openers which help developers use Git better.
-

Inventory Risk Management is an important factor for long-term growth and competitiveness in the medical device industry. Learn five key areas to watch.
-

2023 top 10 medical device commercialization videos cover a variety of medical device commercialization topics ranging from Prototyping Proof of Concepts to Writing Medical Device Manufacturing SOPs.
-
The top 10 Medtech Entrepreneur Webinars of 2023 feature medtech executives, serial entrepreneurs and experts discussing topics for medical device entrepreneurs.
-

Successful medical device exits for startups often involve a combination of other important business factors. These 18 factors are the most important to a successful exit.
-

A medical device commercialization vision helps develop a road map to identify the best path to achieve milestones.
-

33 tips and examples from commercialization experts for manufacturing a medical device.
-

Review of the FDA Selecting a Predicate Device to Support a 510(k) Submission Draft Guidance on Best Practices
-

Medical Device Supply Chain Strategies offers supply chain strategies useful for each product development phase.