Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

When reviewing evidence for a medical device, a single citation can shape an entire submission. In this Bio Break episode, Nick shares a biofilm referencing lesson that has stayed with him since the early 2000s.
-
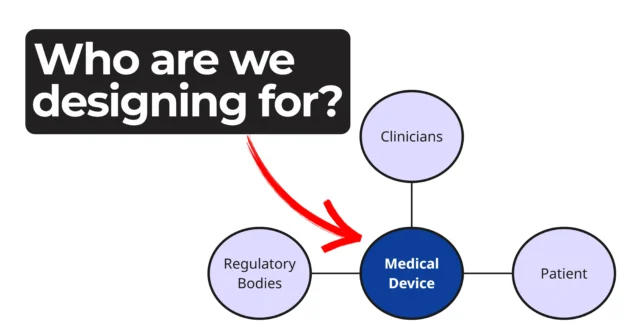
Every phase of a device’s life cycle involves different people with distinct needs—from clinicians and patients to service technicians and regulatory bodies.
-

Ariana Wilson and Mark Drlik explore how teams can reduce the device development timeline without compromising quality or compliance.
-
Understanding medical device classifications is critical for compliance, risk management, and time-to-market success. Whether you’re designing a wearable sensor or a life-sustaining implant, knowing how your device fits into FDA Class 1, 2, or 3 categories is essential.
-

This in-depth drug delivery strategy guide from StarFish Medical explores how to design, develop, and deliver breakthrough drug-device combination products.
-

AI is quietly reshaping how hardware teams work. At StarFish, it’s become a versatile collaborator across the product lifecycle from early sketches to prototype validation.
-

Nick Allan and Nigel Syrotuck share their end-of-summer reading list, featuring FDA regulatory books and PCR memoirs. From navigating regulatory hurdles to celebrating groundbreaking discoveries, their choices show how science reading can be both educational and entertaining.
-

Medical device development is a complex process that requires careful attention at every stage.
-
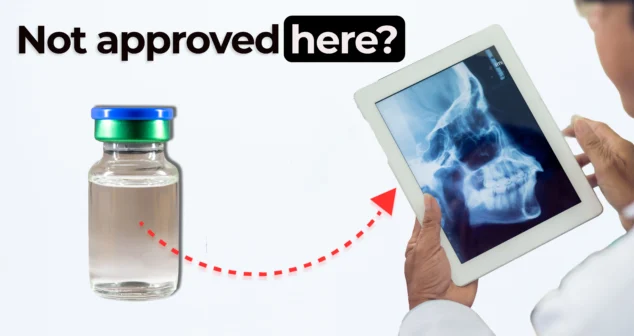
Nigel and Nick explore how contrast agents in imaging support medical device trials and diagnostics. While bones appear clearly in standard X-rays, soft tissues like those in the nasal cavity often require contrast agents to become visible.