Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Learn about the FDA's 2023 guidance on premarket submissions for device software functions and its impact on the medical device industry.
-

The FDA released a new Cybersecurity draft guidance on April 2022. It is intended to replace the current final guidance from 2014 which is well overdue an update. The draft guidance significantly expands requirements for cybersecurity activities and documentation for medical devices. The intention is to align medical device development with current best practices from other industries. This white paper reviews these new requirements and considers their impact on medical device developments.
-

The intent of this checklist is to aid self-assessment of your medical device commercialization readiness relating to regulatory requirements. Compare the answers you provide with stages from the StarFish Product Development Process at the end of the checklist to identify how ready you are for applicable regulatory requirements.
-
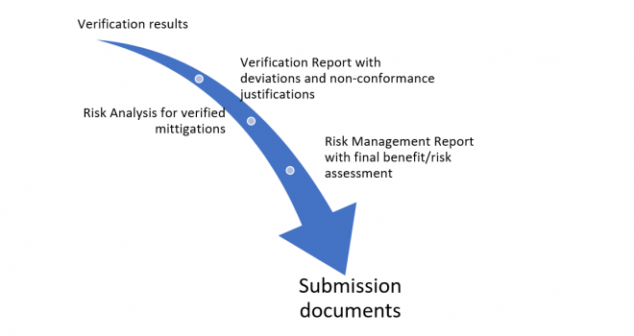
Author describes her experience submitting a medical device application (ventilator) under the Health Canada Interim Order.
-

Health Canada improvements: Getting new devices to market, strengthen monitoring and follow-up and provide more information to Canadians.