Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Risk management is a key element in your innovation process and an excellent skill. Here are five pro tips to managing innovation risk
-

Help guide client and team to achieve project success by considering potential pitfalls and mitigating project risks.
-
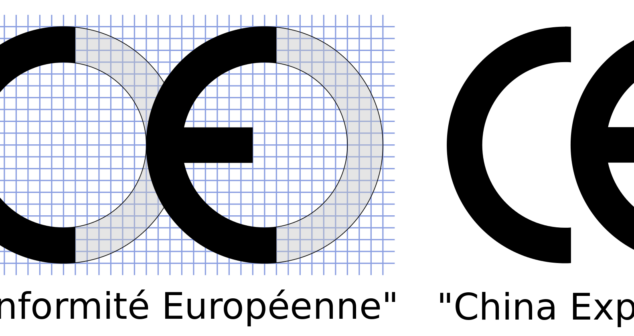
CE vs China Export Mark Explained – Discover meanings, regulations and visual layout of the two marks to avoid costly mistakes.
-

Our most viewed 2016 StarFish Medical videos. Five new entries join this year’s list.
-

2016 summary of most read medical device blogs. This year it will also feel familiar as the top four have appeared previously.
-

Most clicked StarFish newsletter items during 2016. Now’s your chance to see what everyone else was checking out.
-
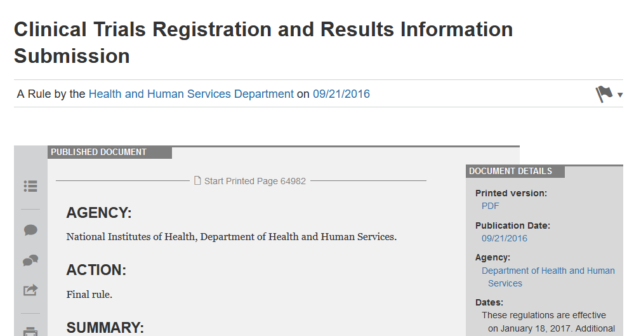
The final rule for clinical trial information reporting published by US National Institutes of Health and DHHS now includes medical devices.
-
Design engineer’s perspective of medical device biocompatibility to help determine if a device needs to be biocompatible.
-

Five points to consider. from the dot-com era to make our medical device digital health strategy bubble proof.