Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

ISO 10993-1 and Biocompatibility impact on Medical Device Plans. Financial impact of additional Medical Device testing will have a cost.
-

Smart approach to Medical Device Product Development. It is very difficult to leapfrog the iterative design process.
-
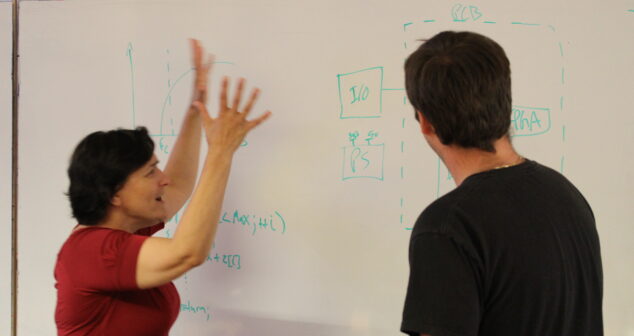
Design reviews are a great way to improve the design of a medical device or product. 8 Proven Best Practices are shared.
-

A Project Management Office (PMO) supports Project Managers and promotes effective medical device development.
-
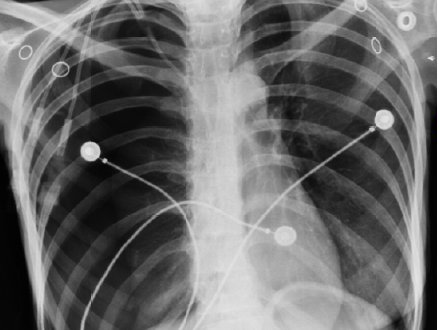
Determining the best radiopaque solution to ensure optimum radiopacity in plastics for your medical device design projects.
-
Medical Device Proof of Concepts (POCs) design best practice – what needs POCing, when to prototype, and how to transition effectively.
-
Product Developers take a complex, technical product from an academic setting and evolve it into a usable device that performs effectively.
-
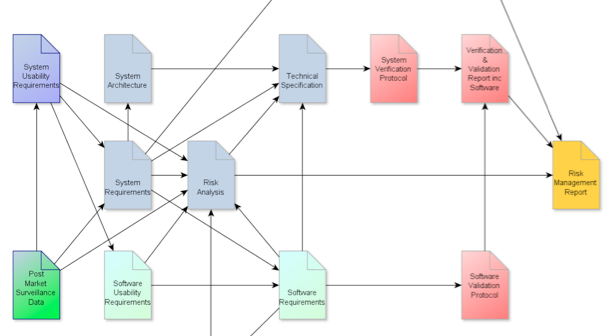
Seven tips for a smooth and successful outcome.to IEC 60601 Edition 3 submissions and electrical safety testing
-
Seed Torrent Brainstorming technique for medical device development delivers better ideas than ideation vs. critical evaluation model.