Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
PCB Design Best Practices that increase the chances of success developing a medical device from Principal designer of printed circuit boards.
-

Engineering expertise in design and development lessons learned in the years since school – both in and out of the medical device industry.
-

Results of StarFish client satisfaction survey with a large group that included customers and prospects using one word descriptions.
-

Six ways to manage and communicate medical device project relationships without losing the essence of the development innovation or wasting money.
-
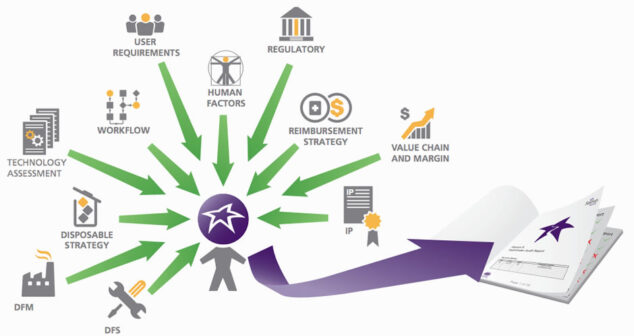
Medical Device Pathfinder process for Intellectual Property, Consumables Strategy, Value Chain and Margin, and Reimbursement.
-
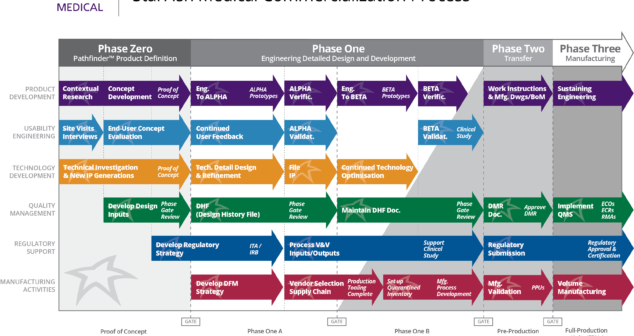
Medical Device Design and Development Process Improvement for Annual Plan: limit improvement efforts, measure them regularly, succeed.
-

The best way to avoid waste is to consider medical device regulatory requirements at a project outset.
-

RoHS 2 and Medical Devices impact design and manufacture. Failure to meet requirements could result in product recalls.
-

7 Digital Hospital Trends to look for in 2014 and keep abreast of technological advancements for surgical skills and clinical relevance.