Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
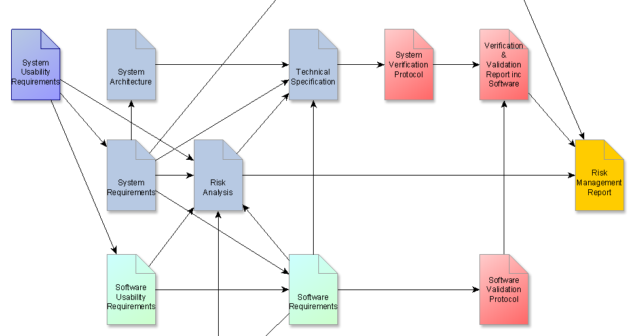
Three medical device design documentation tips for Planning, Input and Architecture when developing early stage medical devices.
-

FDA 510(k) medical device submission tips and advice: being adequately prepared is essential for a smooth submission.
-

Smart approach to Medical Device Product Development. It is very difficult to leapfrog the iterative design process.
-
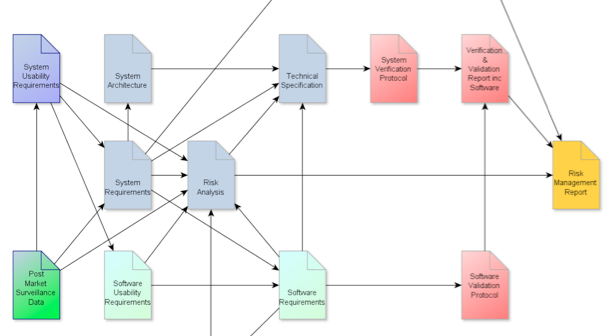
Seven tips for a smooth and successful outcome.to IEC 60601 Edition 3 submissions and electrical safety testing
-

How to manage medical device development expectations to keep development team, investors, and funding agencies all on the same page.