Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
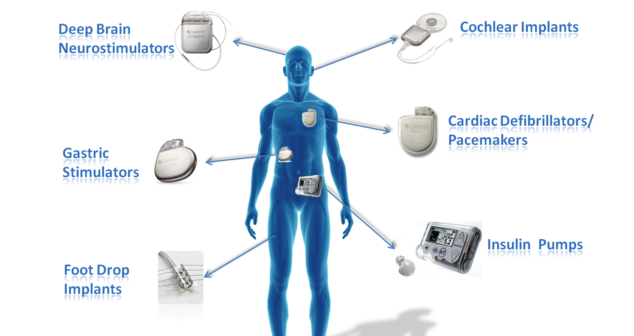
Basics of cyber security as it relates to medical devices, and five tips for developing hack resistant medical devices.
-
Staffing a Medical Device Start-Up: shortlist of job descriptions required to develop a complex, novel medical device.
-

Highlights of 510(k) and CE Marking similarities and differences. Explore the Quality Management System (QMS) and Documentation aspects.
-

Medical Device 510(k) and CE Marking similarities and differences to help companies effectively prioritize their regulatory efforts.
-

Medical Device injection molding partnering tips and considerations: Validation, Cost Benefit Analysis, Vendor Quality Certification.
-

Selecting the right injection molding partner is crucial. Costs and timescales can be a significant part of a project budget. PT 1 of 2
-

Analysis of IEC60601-1-2 (2014) 4th Edition update. Includes examples and impact on medical device regulatory testing and commercialization.
-

Highlights of July and August 2014 medical device FDA guidance documents that reduce regulatory burden without compromising patient safety.
-

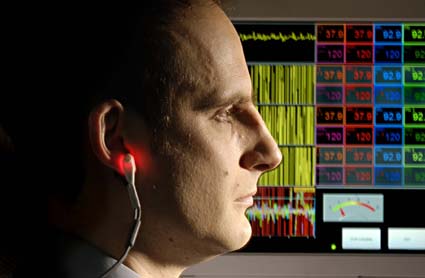
Techniques for IVD algorithm development. IVD algorithm is often required to convert electronic sensor data into a clinical parameter.