Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Embed error detection and correction in the message itself to avoid communication errors in medical device development.
-

Project managers must be vigilant preventing scope creep in software & firmware design, from architecture definition to the production floor.
-

A wonderfully powerful and talented medical device design team can be undermined by omitting a critical expertise – the Tooling Engineer.
-

Reduce Transfer to Manufacturing product risk, cost, and project time with disciplined process, Manufacturing, and Regulatory input.
-
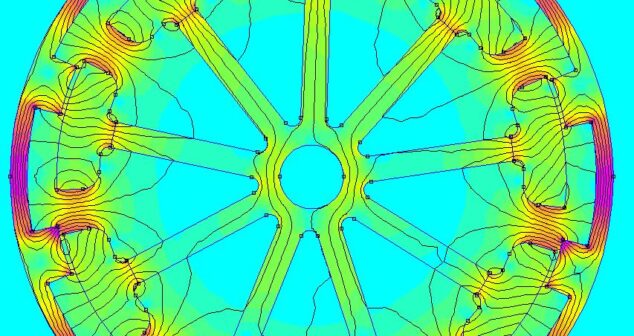
Balancing the analysis vs. resource tug of war in medical device development with 2D simulation.
-
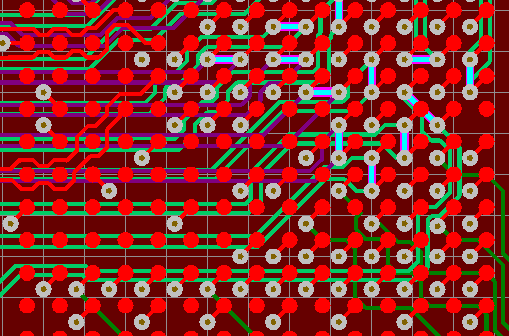
Simulation tools use computers to numerically model the behaviour of a system, particularly an electronic or electromechanical one.
-
Medical vs Consumer Products development employ highly skilled product developers – the difference is in the disposition.
-

How to use conventional vacuum forming techniques and MDF tooling to form foam fast for proof of concept prototypes.
-
Software is not always the answer in medical device design. StarFish blogs about the use of microprocessors in medical device development.Before committing to a design that includes a microprocessor and software, be aware of the regulatory consequences.