Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Round up of Top 10 COVID-19 medical innovations from robotics to COVID 19 POC Diagnostic Tools, PPEs, and Portable dialysis machines.
-
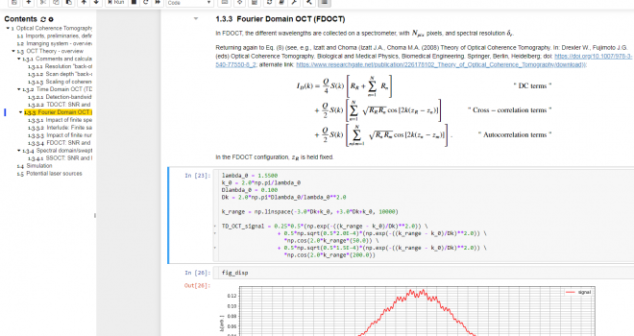
Tips for using Jupyter Notebook in product prototyping creative process and workflow include Jupyter notebook, and Python/Numpy/Scipy stack.
-

Ingress protection for home healthcare medical devices covers the 60601-1-11 collateral standard and what developers should know.
-
COVID-19 Ventilator Design team discuss their top insights and lessons learned from the time constrained, critical ventilator project.
-

Formative Evaluations and Tests details the differences between these two methods, and the appropriate circumstances for their application.
-

COVID-19 friendly brainstorming meetings identifies 5 benefits where virtual brainstorm results outperform in-person brainstorming results.
-

How FDA Breakthrough Devices Program provides advantages for novel devices and can significantly reduce a product’s time to market.
-

Overview of COVID-19 testing authorizations and developments of the COVID-19 pandemic in the United States and Canada.
-
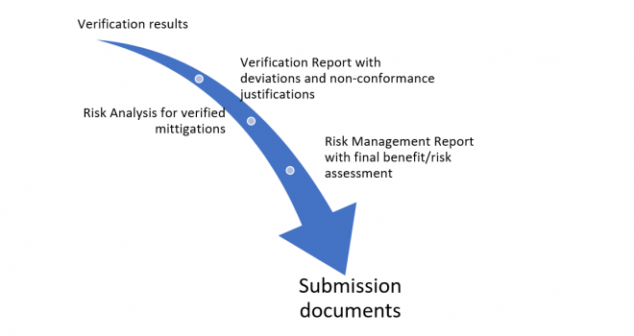
Author describes her experience submitting a medical device application (ventilator) under the Health Canada Interim Order.