Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Effect of Formative Test Fidelity shows the importance of formative testing and its impact on medical device design team usability data.
-

Algorithm Aided Design (AAD) allows for new possibilities and opportunities in the medical market in terms of custom design and 3D printing.
-

Listening Strategy for Startups offers a three-pronged approach to stay competitive within the startup ecosystem.
-

Explanation of changes in medical device symbols and practical implications as a result of the ISO 15223-1:2021 Symbols Update.
-

Outlines how medical device designers can perform in-house Medical Device Drop Test per IEC 60601-1 before third party testing.
-
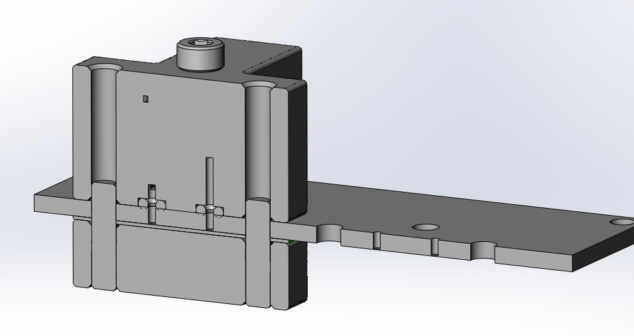
Overview of three physical options for Medical Device Test Fixtures to be used for physical testing fluid connections.
-
Using xFMEA as a Brainstorming Tool uncovers a powerful early-stage medical device design input tool used successfully at StarFish Medical.
-
Considerations when developing a diagnostic product that is intended for use outside a traditional laboratory.
-

Microfluidic Optics explores ways that lenses can be implemented on microfluidic cartridges can play an important role in microfluidic platforms,