Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Tips and a quiz for picking and approving calibration equipment vendors for medical device verification and validation.
-
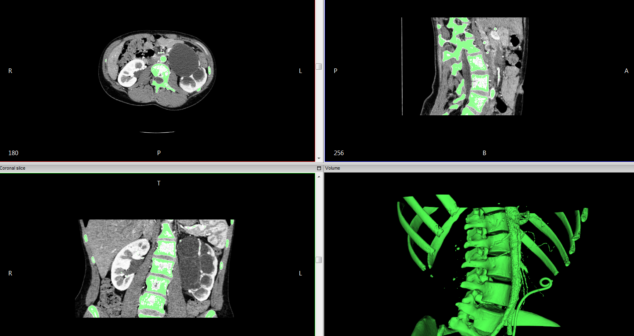
How to create accurate and functional 3D models from patient imaging data. Develop anatomically accurate prototypes for medical device design.
-

Less is More When Designing Medical Device user interfaces, device enclosures, and instructions. Four steps to success!
-

How higher efficiency switching regulators get you more bang out of your buck and maximize efficiency in a switch mode power supply.
-
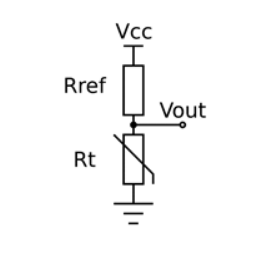
NTC Thermistors as medical device temperature sensors are a good relatively inexpensive option and available in many shapes and sizes.
-

Tips for a productive and successful EMC lab visit and assessment from the StarFish Medical Electrical Engineering team.
-

How to choose a medical device stepper motors, plus different types of stepper motor configurations and how to drive medical device stepper motors properly.
-
Why build a beta medical device? After a medical device receives regulatory clearance, it’s fairly burdensome to make changes. Beta device benefits are explained by experts.
-

One of the key requirements for ISO 60601-1 is the protection against water ingress in Medical Devices. Three areas to watch are discussed in this article..