Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Sterilization is a critical process in the medical device industry as it provides a reliable way to ensure that devices are free from harmful microorganisms when they are used on patients. This blog talks about the categories of sterilization currently used on medical devices in manufacturing settings. It also addresses concerns surrounding the use of ethylene oxide (EtO), an indispensable method for sterilizing heat and moisture sensitive devices.
-
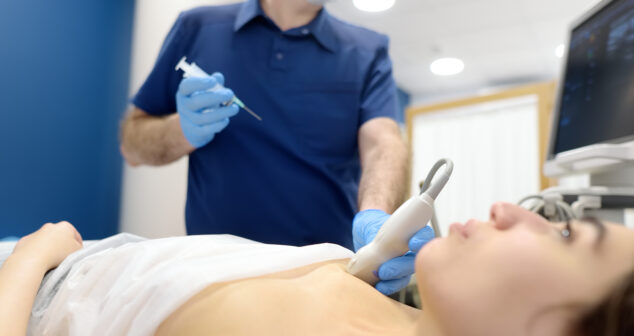
The U.S. Food and Drug Administration (FDA) and the Office for Human Research Protections (OHRP) have released a draft guidance document, Considerations for Including Tissue Biopsies in Clinical Trials, issued in January 2025. It provides recommendations for sponsors, investigators, institutions, and Institutional Review Boards (IRBs) on the safe and ethical incorporation of tissue biopsies in clinical trials.
-
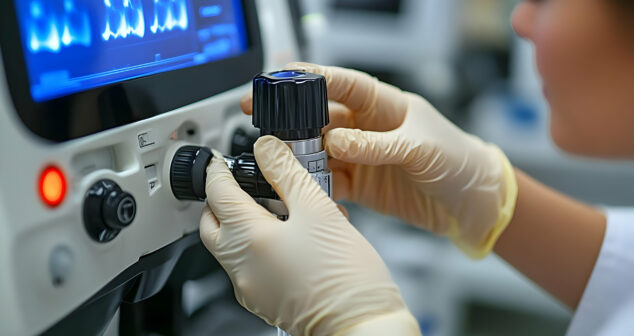
In May 2024, the FDA released an updated guidance document to help manufacturers, third-party service providers, and healthcare facilities determine whether their modifications fall under the category of remanufacturing. This guidance enables these entities to continue ensuring patient safety, regulatory compliance, and the effectiveness of remanufactured medical devices.
-

Common mistakes in medical device projects can create roadblocks that, if left unchecked, can snowball into costly setbacks.
-

ASTM D4169 Options for this standard test method of performance testing shipping containers and packaging systems are explored.
-

What are the most important medical device success factors during development and manufacturing? StarFish employees from QA/RA, NPI, Optics, Computational Analysis, Project Management and Manufacturing answer that question with the factors they deem most important for their area of expertise.
-

This blog explores key highlights of the Chemical Analysis for Biocompatibility Assessment of Medical Devices draft guidance, focusing on its scope, testing methodologies, and recommendations for reporting. In September 2024, the U.S. Food and Drug Administration (FDA) issued a draft guidance titled Chemical Analysis for Biocompatibility Assessment of Medical Devices.
-

Medical Device Commercialization Resolutions include Improved communications, aligning goals, using new tools, going paperless
-
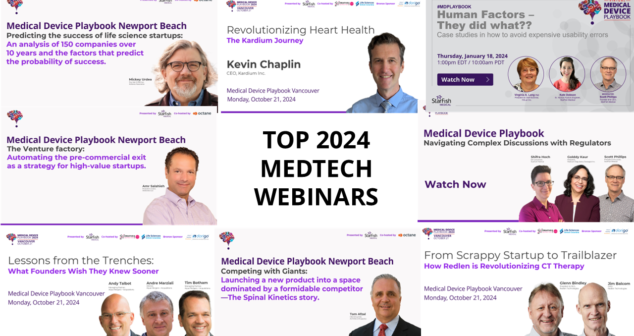
2024 Medtech Entrepreneur Webinars feature both standard expertise webinars and livecast recordings from Playbook events in Vancouver, Canada and Newport Beach, California.