Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Two new blog join eight from last year's top 10 on the list of most-read StarFish medical device blogs and articles for 2022.
-
Medical Device User Testing Tips to get the most out of user testing, a critical part of the medical device design process.
-

Medical device cyber security: 2022 Update includes review of regulations and implications in an ever-evolving landscape of vulnerabilities.
-
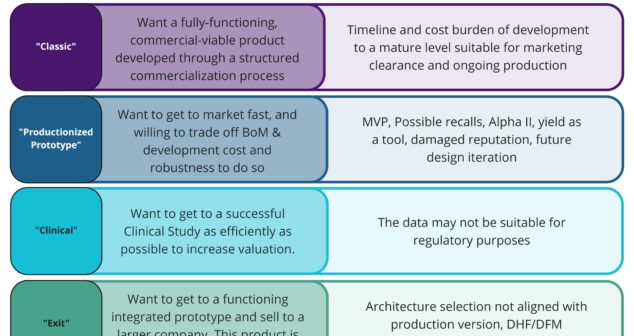
Six common medical device product development destinations and overview of a High Level Program Planning (HLPP) approach.
-
4 insidious hidden costs in medical device development ranging from iterative processes to best practices, and best practices to avoid them.
-

Exploration of the potential in using Medical Device AI with tips on how to avoid pitfalls in AI implementation.
-

XYZs of Medical Device Vibration Testing shares the basics of vibration testing from equipment to key concepts and definitions.
-

Tips and lessons learned on how to manage medtech stakeholder expectations gleaned from 25 years of medical device development.
-
How the FDA Catalog of Regulatory Science Tools can reduce risk in medical devices and explores several tools and categories.