Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Integrating Microfluidics into Biomanufacturing overview and top 4 enabling features of microfluidics for biomanufacturing.
-
The do's and don'ts of running Industry-Integrated Engineering Courses including University of Victoria's BME401C collaboration with StarFish.
-

Many techniques can be employed in order to simplify formal verification testing. These techniques expedite the product development process and reduce cost….
-

2022 New Year's Resolutions from StarFish employees offer a glimpse of our culture and invite kindred spirits to join us in the new year!
-
Overview of the FDA action plan for AI/ML in software as a medical device (SaMD) and list of best practices.
-
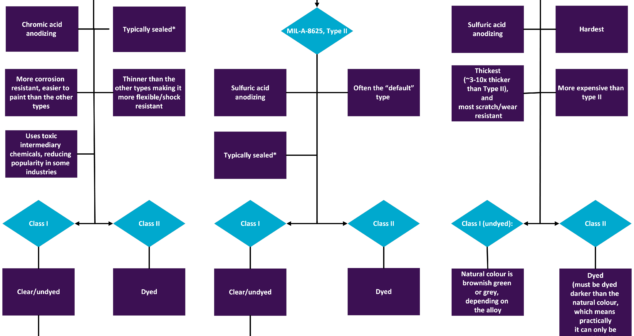
Features and benefits of different Mil-Spec Anodizing Types and Classes of anodized coatings in North America.
-

StarFish Medical content experts offer up fifteen favorite reads from 2021, covering business, fiction, biographies, self-improvement.
-

2021 top medical device commercialization videos cover 2021 updates, optimizing founder value, manufacturing for NPI, IVD insights. and more.
-
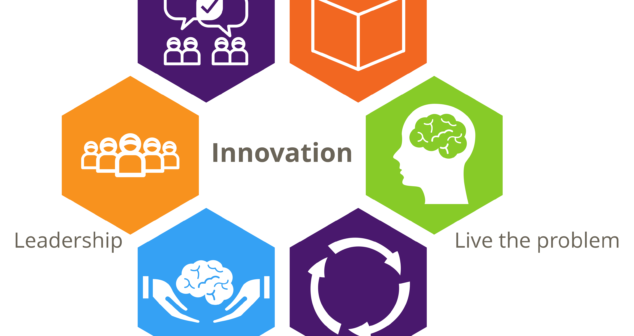
Often medical device innovation is blocked because the wrong ingredients were present. Discover key components driving innovation.