Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

NPI team's top tips for production readiness based on their collective experiences transferring medical devices from design to production.
-

PFMEA prevents causes rather than detecting them (or the effects of failures), and should be incorporated into medical device design.
-

Any medical device first to market production release strategy that minimizes time requires an honest examination of the phase-gate process.
-

Reliability testing is an effective money saving NPI tool that helps produce high-quality medical devices which work consistently.
-

The Bleeding Edge explains how 510K cleared medical devices are clinically proven to improve patient outcomes and save lives.
-

The book Bad Blood eloquently demonstrates the importance of properly developing medical devices under ISO 13485.
-
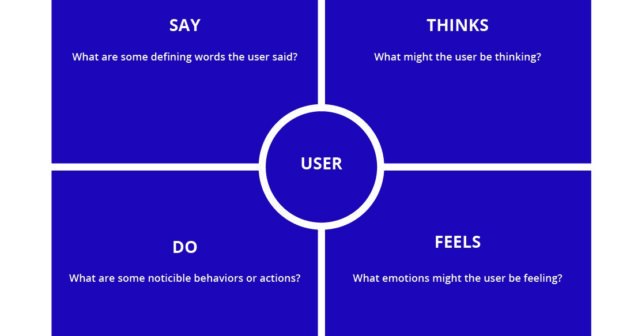
Empathic Design paying attention to what female users say, think, do, and feel, gives a holistic understanding of user desires and needs.
-

Alarm fatigue is influenced by a variety of factors. End user and patient behaviours are most identifiable through procedural observation.
-

Setting up observation sessions can be quite time consuming. Three tips to make the most of your time and results.