Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

2021 top medical device commercialization videos cover 2021 updates, optimizing founder value, manufacturing for NPI, IVD insights. and more.
-
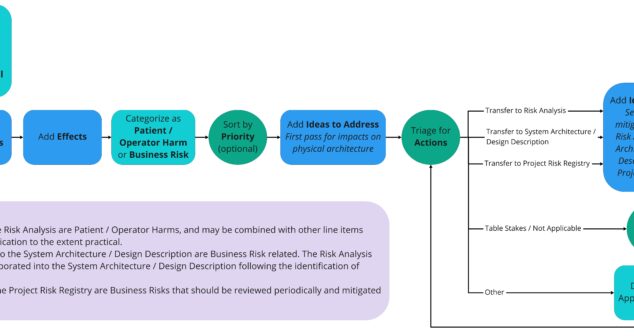
Using xFMEA as a Brainstorming Tool uncovers a powerful early-stage medical device design input tool used successfully at StarFish Medical.
-

Ensuring new hires feel connected to the team and engaged in their position is critical. Learn five considerations for successful onboarding.
-

Returning to Work Safely during COVID discusses documents and programs from Intertek, BSI and TÜV that assist ISO PAS 45005 compliance.
-

When COVID is Under Control – StarFish Medical employee choices for their first activities when COVID restrictions are eased.
-

Mechanical Engineering Team lead shares his response to students who ask him what the ideal medtech co-op looks like.
-

Questions and resources to help you find the right medical device development partner for your unique needs and requirement.
-

Working at StarFish – Guess the top 9 surprises our employees noticed when they first started working at StarFish and still notice after many years.
-

Medical Device Case study example where "dig deeper" – IEC 60601 saved the author from potential problems later down the road.