Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Seven Medical Device Commercialization Polarities with tips on how to manage them throughout the development and commercialization process.
-

Favorite medical device development information resources and tips for getting unstuck with a new question or challenge.
-

Six BioManufacturing Trends to Watch in 2022 and the future of BioManufacturing analyzed by Bioscience and medical device experts.
-

Many techniques can be employed in order to simplify formal verification testing. These techniques expedite the product development process and reduce cost….
-
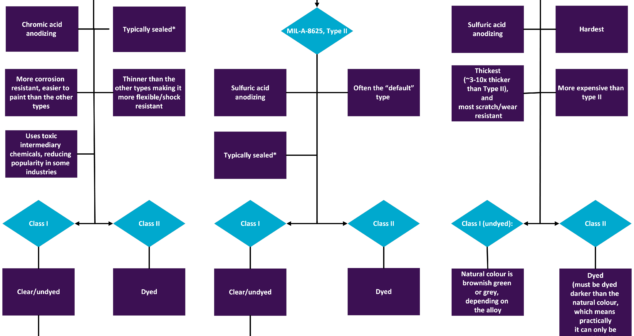
Features and benefits of different Mil-Spec Anodizing Types and Classes of anodized coatings in North America.
-

2021 top medical device commercialization videos cover 2021 updates, optimizing founder value, manufacturing for NPI, IVD insights. and more.
-

2021 “most read” list: Electrical engineering and regulatory articles tie for the most popular blog topics.
-
Optical Engineer shares key elements that impact image quality when choosing a Medical Device Camera Sensor.
-
Medical Device New Product Introduction (NPI) & Commercialization lessons for medical device strategy, design, development, and NPI success.