Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
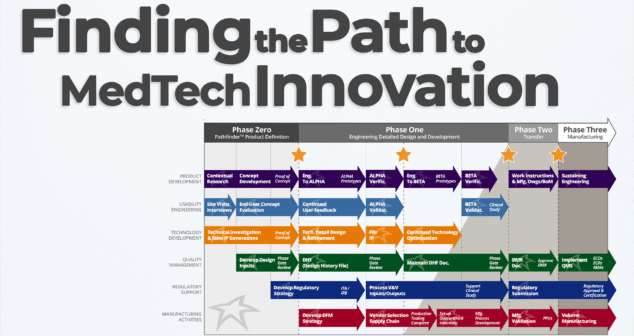
Nick and Joris explore one of the most dynamic early-phase services at StarFish Medical: the Pathfinder Program. If you're a medtech innovator with a promising concept or prototype, Pathfinder helps you identify the right path forward—before you invest millions in development.
-

Nick and Joris explore the wide world of ablation technologies—unpacking how each approach works and what it’s best suited for.
-
Nick Allan and Joris van der Heijden dive into one of the most impactful trends in modern medtech: minimally invasive surgery. Ablation technology plays a crucial role as hospitals and healthcare providers aim to reduce patient recovery times and overall system strain.
-
Fluorescence Imaging in Medical Devices outlines medical applications and examples of devices that use fluorescence for imaging.
-
Computer Vision for Medical Devices is constantly evolving and incorporating new techniques and technologies as they emerge.
-
In this episode of Bio Break, Nick and Joris dive into the rapidly growing field of minimally invasive medical technologies, focusing on…
-
In this episode of Bio Break, Nick and Joris dive into one of the most astonishing—and real—medical innovations we’ve ever come across: osteo-odonto-keratoprosthesis. Or, as Nick quickly dubs it, “tooth in eye surgery.”
-
Ariana Wilson and Mark Drlik break down a powerful visual framework for understanding what makes a medtech product, and the company behind it, truly successful. Using a triple Venn diagram, Mark explains how strategic alignment across feasibility, viability, and desirability can drive better product outcomes and business success in the medical device industry.
-

What are the real costs of developing a medical device? In this episode of Bio Break, Nick and Joris dive into one of the most frequently asked questions they hear from clients: How much does it cost to develop a medical device?