Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

In the highly regulated world of medical device development, ensuring product safety, quality, and compliance is essential. One critical yet often overlooked aspect of this process is test method validation (TMV) in medical device development.
-

A structured, well-documented design review process is a critical component of successful product development, particularly in the medical device industry.
-

In medical device development, we deal with complex projects that span multiple disciplines, timelines, and regulatory gates. It’s a constant balance between moving fast enough to innovate, but slow enough to stay compliant.
-

Ariana and Mark walk through FDA-approved options and explain how to select the right one for your product. From metals to plastics and electronics, not all devices can handle the same process.
-
Many clients now request their devices to look and feel like Apple products. But achieving that level of simplicity and elegance is not as easy as it seems.
-

You have a great MedTech innovation idea and are trying to decide whether to build a team to commercialize a medical device…
-
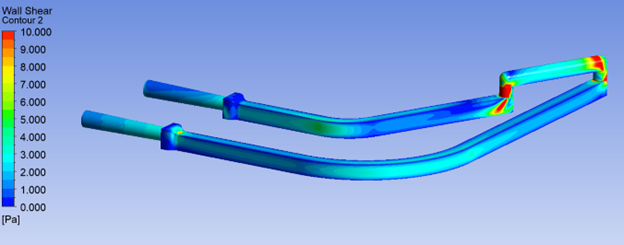
The impact of shear stress is critical to effectively design medical devices that handle biological fluids such as proteins or cell culture media. For example, non-physiological shear stress (NPSS) on blood is a key factor because hemolysis (cell rupture) could occur due to accumulated stress.
-

The costs of early-stage medical device development in North America and Europe continue to rise. Increasing technical complexity and the compounding costs of nonclinical and clinical evaluations are driving this trend.
-

Today, 85% of the top 50 healthcare companies use Computational Modeling and Simulation (CM&S) to develop their products and processes. Whether it’s refining overall device parameters or optimizing critical requirements, engineering simulations help reduce development timelines and enhance design exploration.