Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
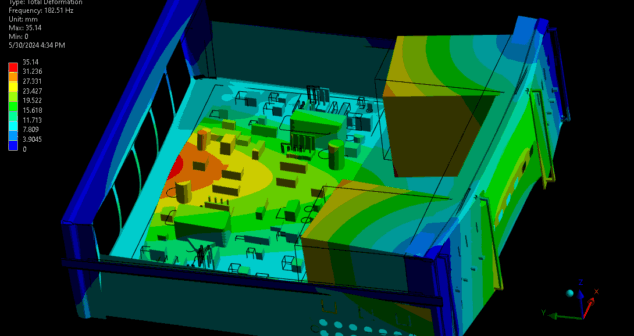
The impact of vibration on medical devices can be understood through a combination of vibration analysis and testing. This article reviews medical devices most susceptible to vibration, provides basic theory and methods of vibration analysis, and summarizes the benefits of using modal analysis when developing medical devices.
-
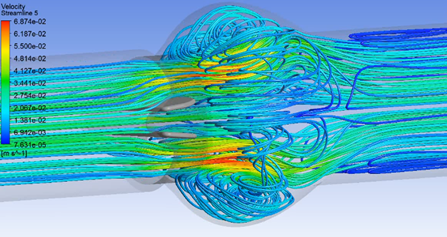
Simulation technologies are revolutionizing the field of heart valve repair and replacement, offering unprecedented insights and capabilities. Enhancing our understanding of valve mechanics, improving device design, optimizing surgical techniques, and enabling personalized treatments, simulations are paving the way for better cardiovascular care and patient outcomes. This article explores the transformative role of heart valve computational simulation in understanding, and treating heart valve diseases.
-

Four areas that can make or break a new medical device development project from the start. Experts identify actions and offer advice that helps ensure new medical device projects start on the right foot.
-

Summary of nine-step process for developing and assessing the credibility of CM&S for regulatory submissions in the FDA Guidance Document “Assessing the Credibility of Computational Modeling and Simulation (CM&S) in Medical Device Submissions (17th November 2023)”.
-

Speed to market is a crucial part of medical device success. Experts draw upon their experience with hundreds of medical device products for proven tips and recommendations that increase medical device speed to market without sacrificing safety or quality.
-

Engineers, regulatory, manufacturing and optics experts share their experiences and lessons learned commercializing hundreds of medical devices with optics components and interacting with optics engineers.
-

SBOM Analysis and Value covers the FDA “Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions guidance, role of Software Bills of Materials (SBOMs), and how to create them.
-

2024 FDA guidance on medical device cybersecurity covering risk management, design controls and software validation is explained in this article. Cybersecurity, Risk Management, Secure Product Development Framework (SPDF) are also covered.
-
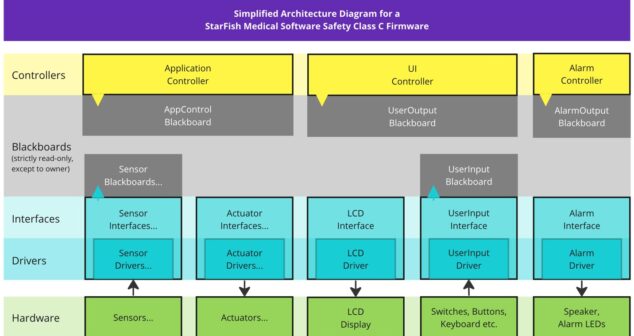
How to develop Class C Firmware for medical devices and implement Segregation in compliance with the IEC 62304 Standard.