Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

FDA inspection outcomes from 3 very different inspection preparation strategies with lessons learned from the inspections.
-
The two key variables in rapid silicone prototyping are silicone material selection and moulding strategy.
-

4 ways (with related principles) to make your medical device jig design process more effective. Jigs help us conduct tests and collect data.
-

Adaptive studies can be especially useful in the pivotal stage if there are uncertainties about one or two aspects of the study.
-

FDA final rule on symbols is an important step towards global medical device harmonization and hopefully for a decrease in manufacturing costs as well.
-

Review of sections 4, 5 and 6 of ISO 13485:2016 and the major changes from the 2003 version with potential impacts on QMS.
-

FDA inspection of our Quality Management System (QMS) and 2 medical devices that are contracted to StarFish Medical manufacturing.
-
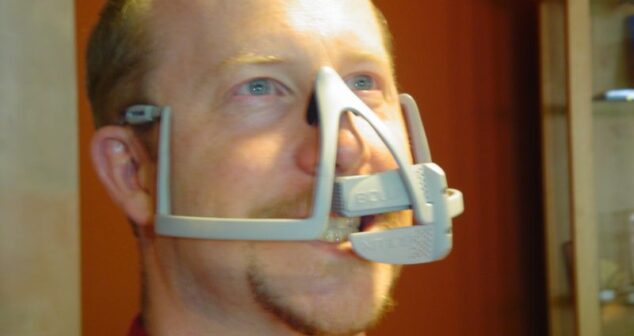
Exploration of applying IEC-62366-1:2015 or human factors engineering to the design of medical devices compared to the FDA guidance document.
-

The power of initial concept design sketches – It’s imperative they reflect all of the requirements, and not just the marketing ones.