Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Medical Device Design for the Developing World – why are there so many nonfunctional and broken medical devices in low-income regions?
-

Microfluidics for molecular and serological diagnostic tests enable shorter turnaround times, high accuracy results, and lower cost per test.
-
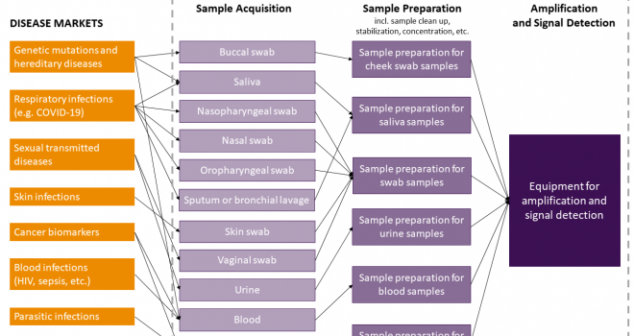
Designing Point of Care Diagnostics to be truly universal and agnostic to the sample matrix will certainly provide a huge advantage.
-
Simulated Fluids for POCT Development enable testing using a liquid that mimics the critical properties of the desired clinical sample.
-
Efficient diagnostic device design requires having a good, well-defined Target Product Profile (TPP) going into the development cycle.
-
Medical device color management overview of why colour-rendering accuracy is critical for LEDs CRIs and Full-Colour Imaging.
-

Verify Environmental stress cracking (ESC) quickly, it's one of the most common failure mechanisms in parts made of polymeric materials.
-

Medical Device Case study example where "dig deeper" – IEC 60601 saved the author from potential problems later down the road.
-

FDA ASCA Pilot Program is expected to bring medical devices to patients and users more efficiently by using ASCA-accredited labs.