Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Pantone in Medical Device Development discusses Panton application and use, and why it is important to precisely communicate colors.
-

5 reasons Human Factors remain important in medtech despite technological advancements like Artificial Intelligence (AI).
-

Explore ways to leverage PCB mounted Microfluidic components for compact, reliable interaction with microfluidic cartridges.
-

Medical device regulatory and QMS hidden costs can often be avoided when projects are well defined early to understand and know the costs up front.
-

Tips and examples for Product Definition (Phase Zero) of medical device commercialization process.
-

Analysis of FDA draft guidance "Decentralized Clinical Trials for Drugs, Biological Products, and Devices."
-
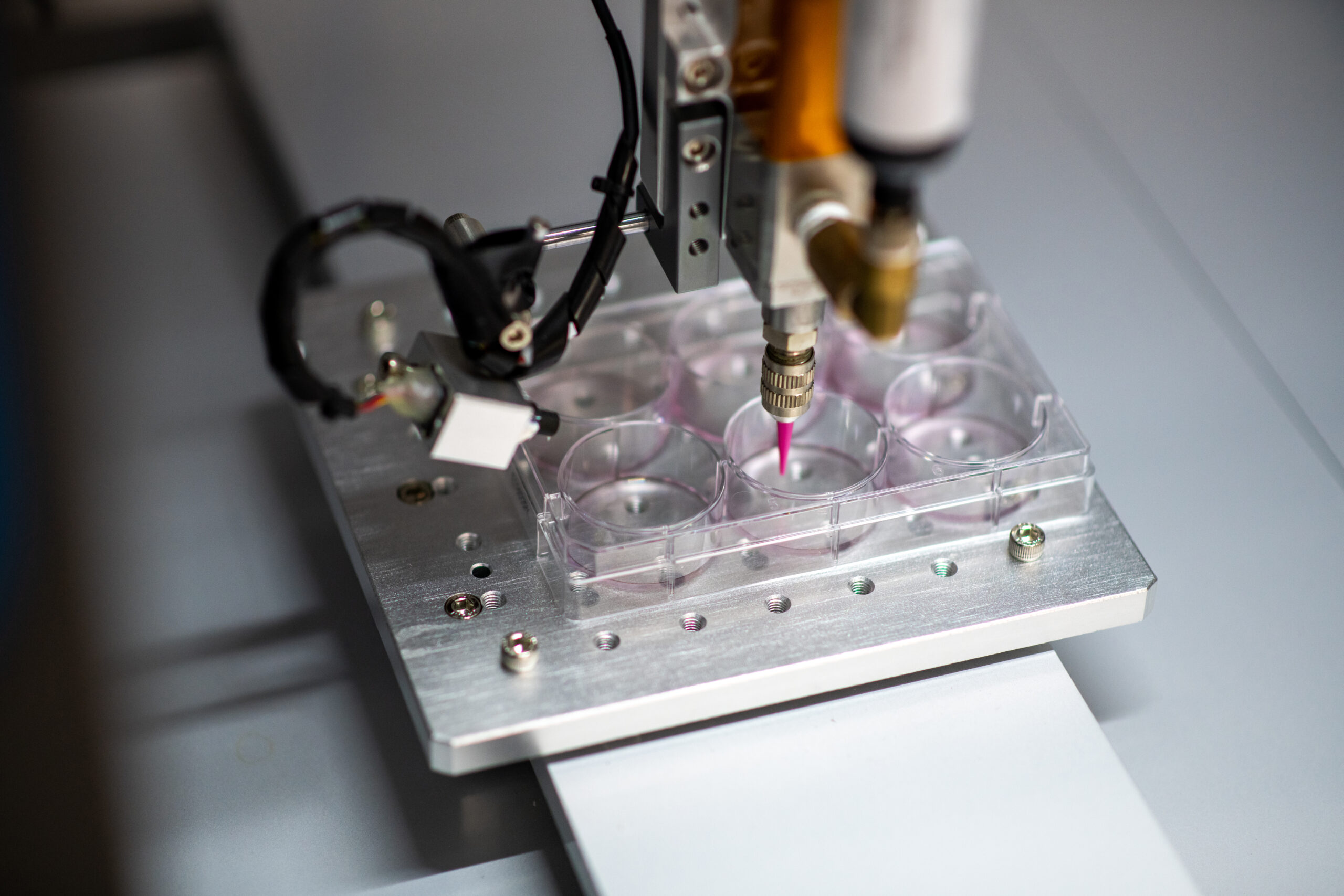
Bioprocessing (biologics manufacturing) 4.0 requires innovation in bioprocess equipment design, control and integration.
-

Systems engineering can help early-stage biotech companies overcome challenges associated with technology transfer.
-

Understanding lipid nanoparticles and microfluidics, the technology behind the unprecedented speed of vaccine production.