Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
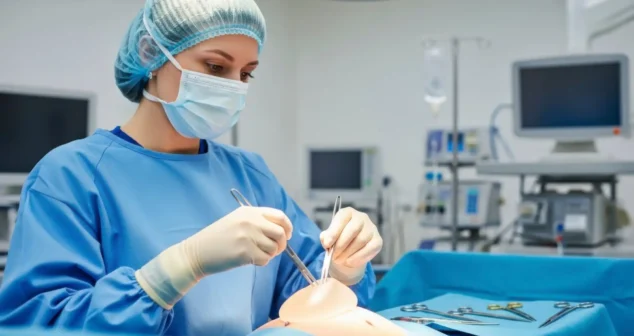
The simplest and least expensive way to train users of medical devices is to ask them study the Instructions for Use (IFU) beforehand.
-

Pre-clinical studies and early-stage trials are some of the most challenging — and expensive — milestones in medical device development. Yet, many teams encounter avoidable setbacks that could have been prevented with strategic foresight and practical lessons learned from experience.
-

Experts discuss 3 pre-clinical pitfalls that could derail your drug delivery device. One small oversight could cost you millions in clinical delays.
-

Areas to be considered in Summative / Human Factors Validation planning, include test environment, and user profile review.
-

Reduce medical device program risks with smart testing strategies – a systematic approach to device testing.