What seasoned medtech developers would do differently — and why it matters
Pre-clinical studies and early-stage trials are some of the most challenging — and expensive — milestones in medical device development. Yet, many teams encounter avoidable setbacks that could have been prevented with strategic foresight and practical lessons learned from experience.
In the webinar “Pre-Clinical Lessons We Wish We’d Known,” three experienced medtech professionals — Joris van der Heijden (Concept Development Lead), Paul Hulme (Human Factors Engineer), and Nick Allan (Bio Services Manager) from StarFish Medical — share candid stories, real-world challenges, and the pivotal pre-clinical insights they’ve gained over decades of medical device development.
Key Takeaways from the Webinar:
- Design with the drug container in mind — Why selecting or accommodating the right primary drug container is critical for pharma partnerships and market acceptance.
- Minimizing use errors through user-centered design — How real-world user behavior and stress conditions impact device performance, and why early usability testing is essential.
- Lessons in IFU reliance and training — Why you can’t count on users reading instructions, and how hands-on training and competency checks can mitigate risk.
- Packaging and labeling pitfalls — Surprising findings on what users actually notice on packaging (and what they don’t) and how simple design changes can improve safety and compliance.
- The risks of last-minute design changes before trials — A cautionary story from clinical research where small hardware adjustments led to major setbacks.
- Pre-clinical validation strategies that work — How simulation models, imaging studies, and stepwise testing can reduce uncertainty before entering costly clinical trials.
Whether you’re an engineer, program manager, clinical lead, or regulatory professional, this webinar offers practical, hard-earned advice to help you avoid delays, reduce risk, and position your medical device for clinical and market success.
Speakers
- Joris van der Heijden, Concept Development Lead, StarFish Medical
- Paul Hulme, Human Factors Engineer, StarFish Medical
- Nick Allan, Bio Services Manager, StarFish Medical

Pre-Clinical Lessons We Wish We’d Known
Insights to help you avoid costly mistakes — before clinical trials begin.
Related Resources

A structured, well-documented design review process is a critical component of successful product development, particularly in the medical device industry.

In medical device development, we deal with complex projects that span multiple disciplines, timelines, and regulatory gates. It’s a constant balance between moving fast enough to innovate, but slow enough to stay compliant.
Ariana and Mark walk through FDA-approved options and explain how to select the right one for your product. From metals to plastics and electronics, not all devices can handle the same process.
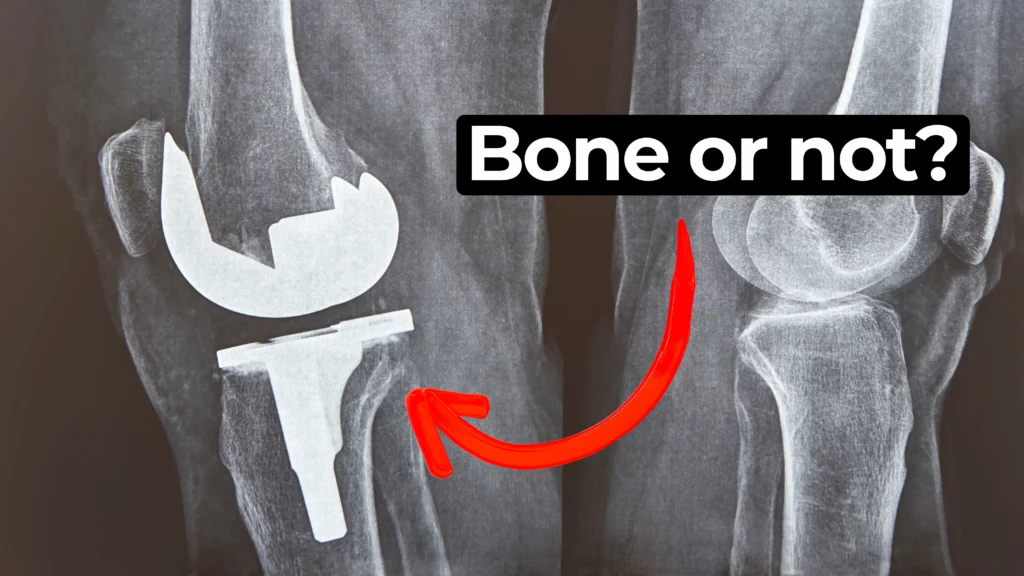
In this episode of MedDevice by Design, Ariana and Mark dive into the biomechanics and materials science behind osseointegration for implants.
